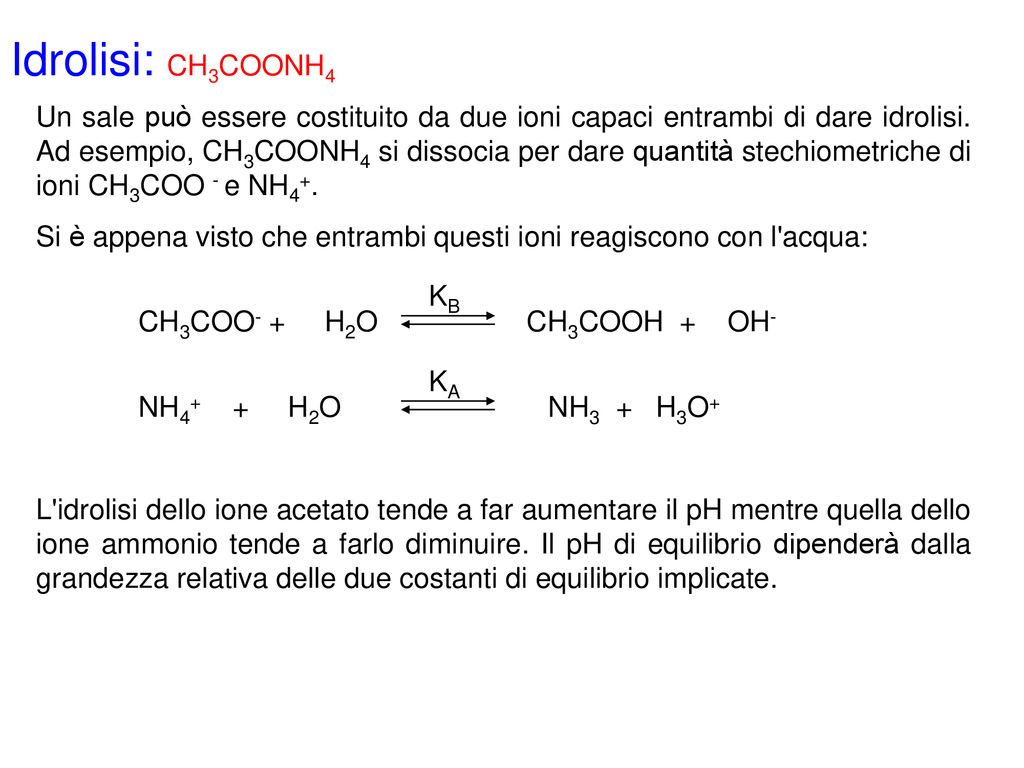
Identity correct statement (a)degree of hydrolysis decrease on doubling the concentration of aqueous solution of CH3COONH4 (b)for 1M CH3COOH pH=pKa/2 (c)salt hydrolysis depends on size of atom (d)all

Calculate the percentage hydrolysis & the pH 0.02 M CH3COONH4. Kb(NH3) = 1.6 × 10^-5, Ka(CH3COOH) = 1.6 × 10^-5.

WHICH OF THE FOLLOWING INCREASING ORDER OF PH OF .1 M SOLUTION OF THE COMPOUND A-HCOONH4,B-CH3COONH4 - Brainly.in
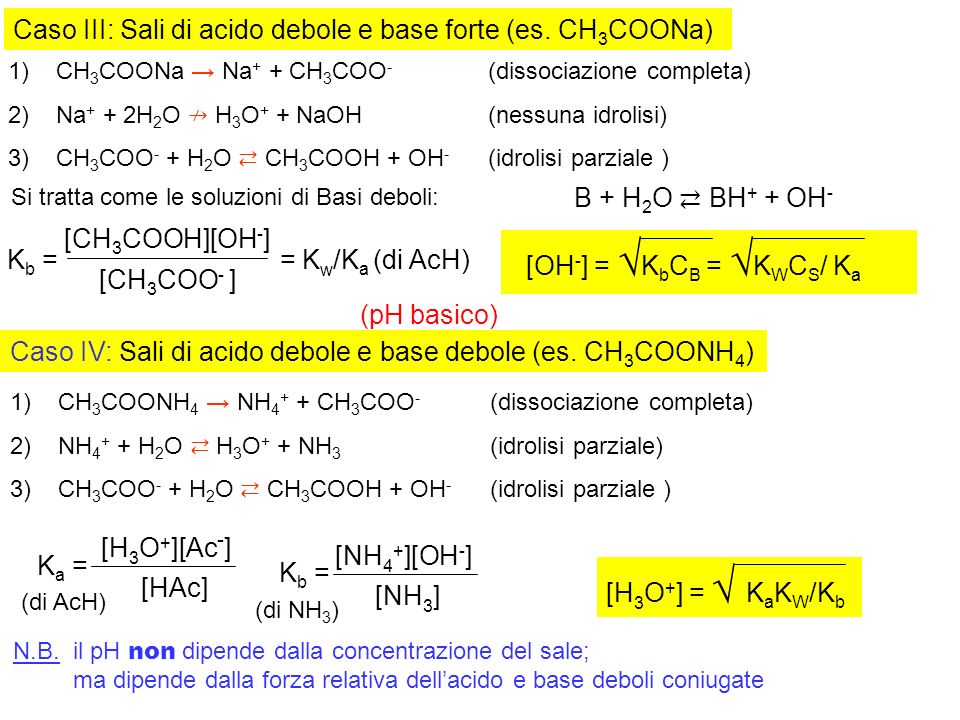
Il pH della soluzione di un sale Caso I: Sali di acido forte e base forte (es. NaCl) 1)NaCl → Na + + Cl - (dissociazione completa) 2)Na + + 2H 2 O ↛ H. - ppt scaricare
If the pKa of CH3COOH and pKb of NH4OH are the same as 4.76, what is the pH of an aqueous solution of ammonium acetate? - Quora

Calculate the percentage hydrolysis & the pH 0.02 M CH3COONH4. Kb(NH3) = 1.6 × 10^-5, Ka(CH3COOH) = 1.6 × 10^-5.

Calculate the percentage hydrolysis & the pH 0.02 M CH3COONH4. Kb(NH3) = 1.6 × 10^-5, Ka(CH3COOH) = 1.6 × 10^-5.

Which of the following solutions will be acidic?(1) 0.1M FeSO4 (2) 0.1M (NH4)2SO4 (3) 0.1M CH3COONa (4) 0.1M NH4OH
Calculate the extent of hydrolysis and the pH of 0.1 M ammonium acetate Given that. - Sarthaks eConnect | Largest Online Education Community

Calculate the percentage hydrolysis & the pH 0.02 M CH3COONH4. Kb(NH3) = 1.6 × 10^-5, Ka(CH3COOH) = 1.6 × 10^-5.
8. Which of the following increasing order of pH of 0.1M solutions of the compound A HCOONH4 B CH3COONH4 C CH3COONa D NH4Cl is correct

Hitunglah pH larutan CH3COONH4 0,5 M, jika diketahui Ka CH3COOH =10 pangkat min 11, Kb NH3 = 10 pangkat - Brainly.co.id

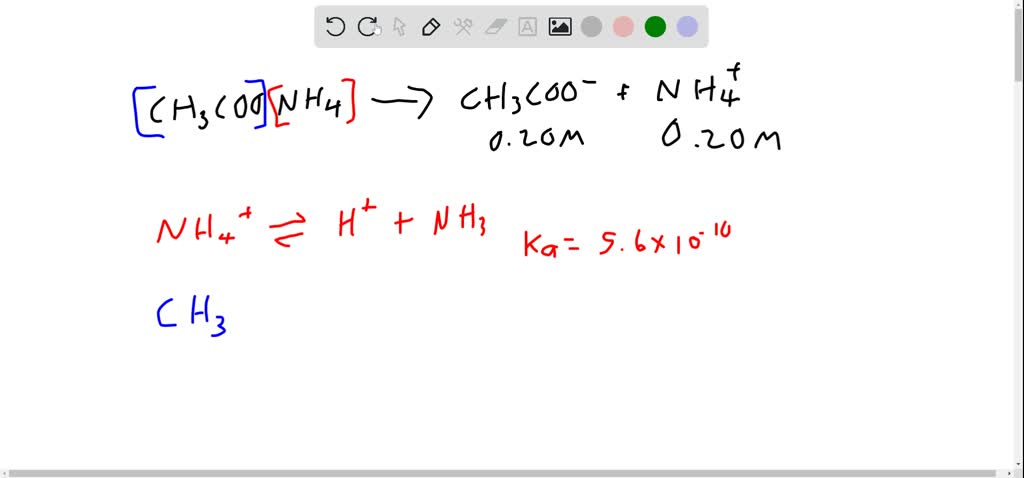
![What is the pH of 0.02M CH3COONH4 ?[ Ka = 1.8 × 10^-5,Kb = 1.8 × 10^-5 ] What is the pH of 0.02M CH3COONH4 ?[ Ka = 1.8 × 10^-5,Kb = 1.8 × 10^-5 ]](https://haygot.s3.amazonaws.com/questions/1838174_1312507_ans_dfcdd24825da474ab196153f6eb7756a.jpg)