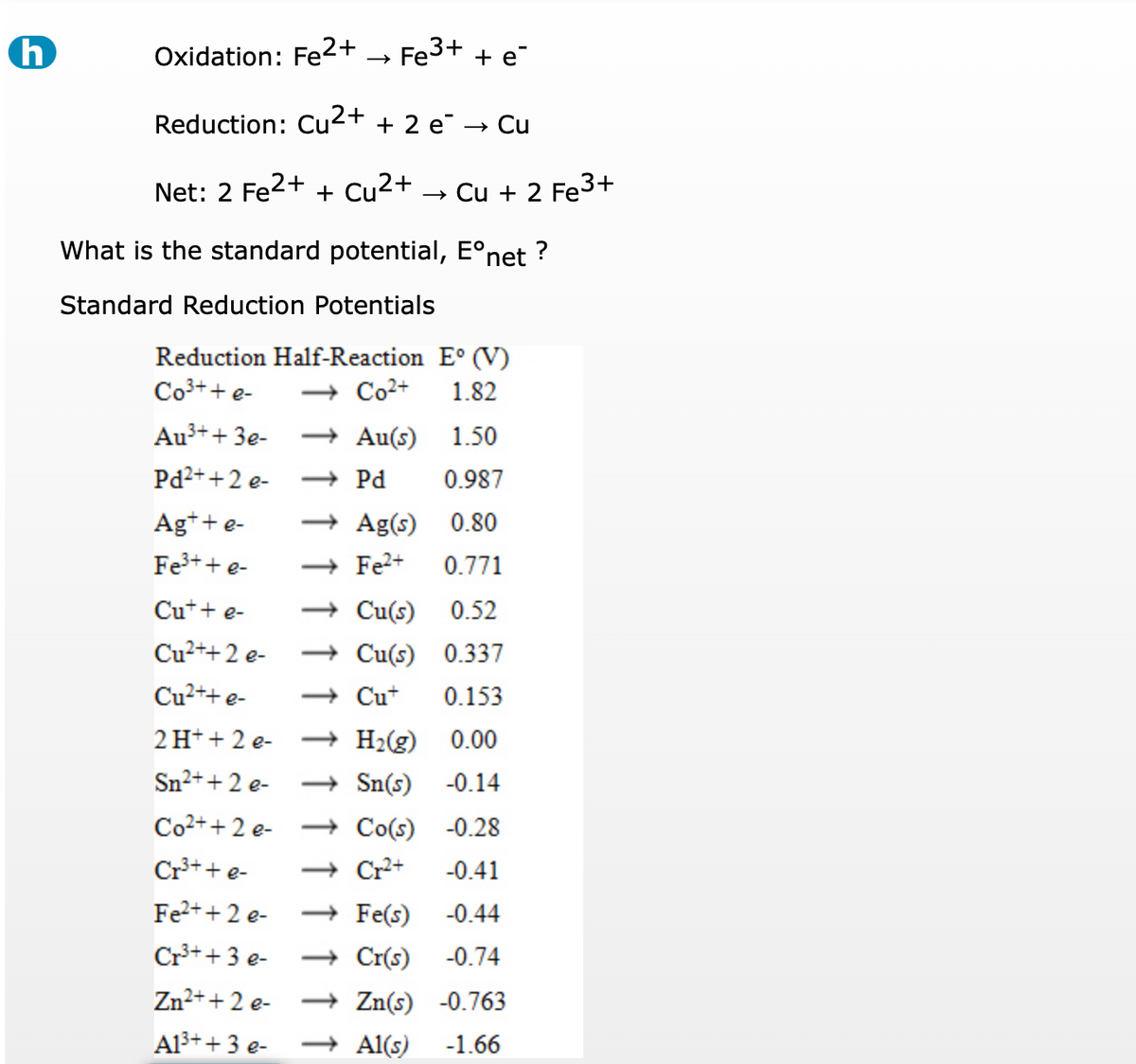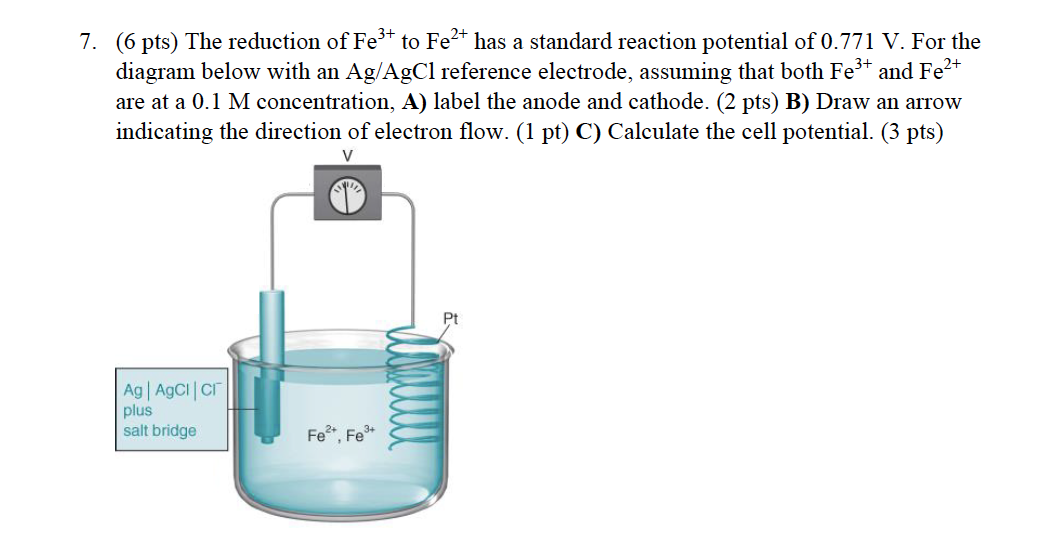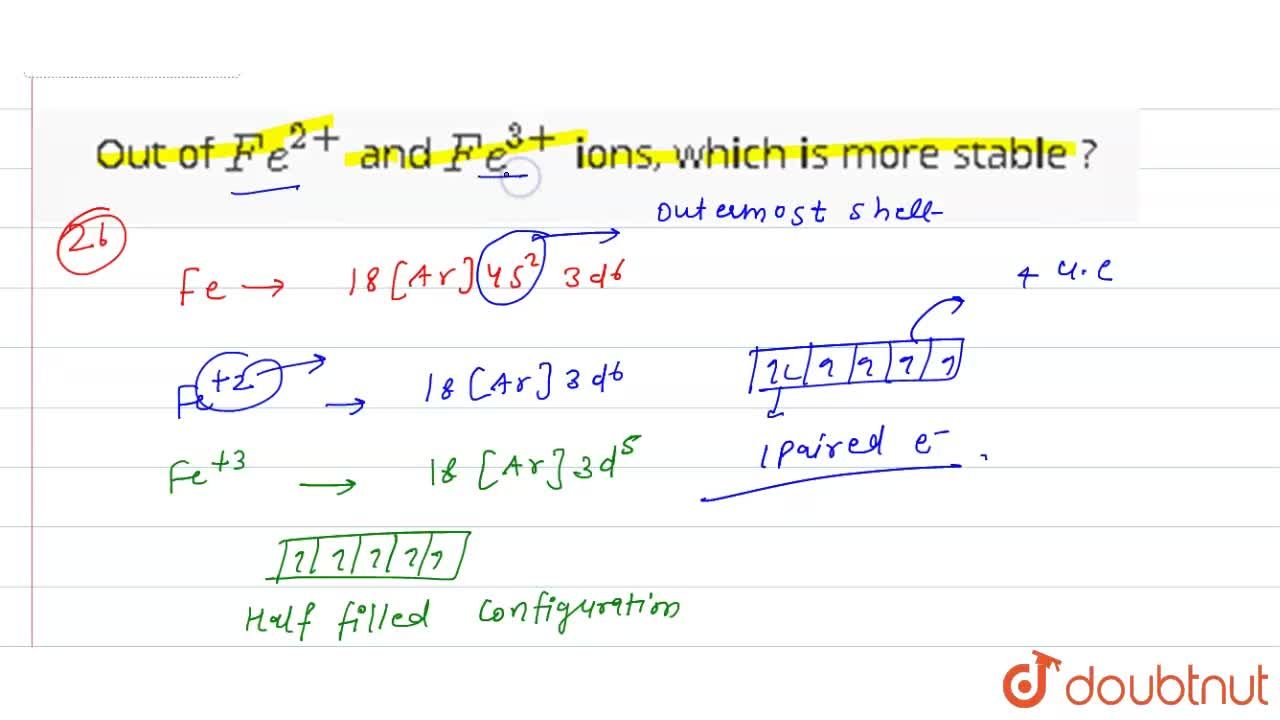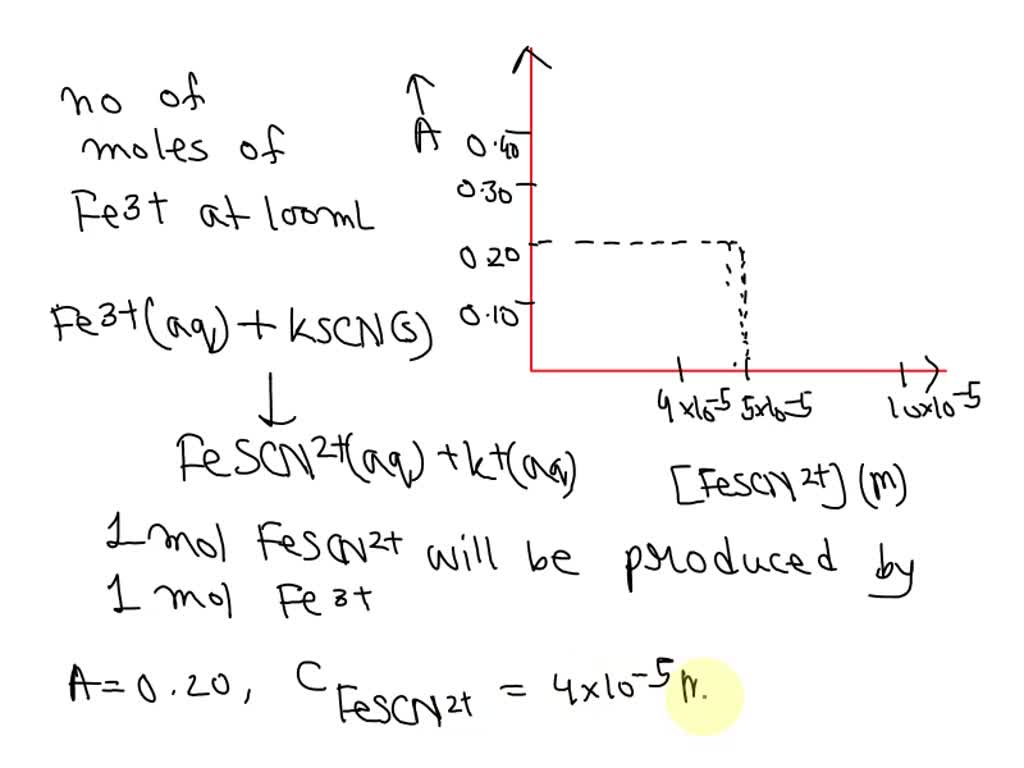
SOLVED: Fe3t(aq) KSCN(s) FeSCN2tC "(aq) Kt(aq) To determine the moles of Fe3t(aq) 100. mL sample of an unknown solution; excess KSCN(s) is added to convert all the Fe3+ (aq) into the dark

The molar ratio of Fe^2 + to Fe^3 + in a mixture of FeSO4 and Fe2(SO4)3 having equal number of sulphate ions in both ferrous and ferric sulphates is:

SOLVED: The chemical formula for iron(III) sulfide is Fe3(SO4)2 FezS3: Fe3(SO3)2: Fez(SO3l3 Fez(SO4)3:

Multimedia Video Player | Electronics | Car Radio | Hyundai | Santa - Car Multimedia Player - Aliexpress

Ascorbate: the phantom menace? Catalytically active ferric (Fe3+) and... | Download Scientific Diagram

EPR spectra of Fe3+ and Fe3+–enterobactin bound to FrpB. EPR spectra... | Download Scientific Diagram

FE3.jpg - KAHU Nature Photography, , a selection of beautiful New Zealand landscapes and bird photographs plus workshops, photo safaris and more

Given electrode potentials are : Fe^3 + + e^- → Fe^2 + ; E^ = 0.771 V I2 + 2e^- → 2I^ ; E^ = 0.536 V Find the E^ cell for the cell reaction: 2Fe^3 + + 2I^ → 2Fe^2 + + I2 is :