![SOLVED: Calculate [H3O+] for each solution. there a different parts and values to this question. Each value is a part. pH = 8.35 to [H3O+] = ? M pH = 11.23 to [ SOLVED: Calculate [H3O+] for each solution. there a different parts and values to this question. Each value is a part. pH = 8.35 to [H3O+] = ? M pH = 11.23 to [](https://cdn.numerade.com/ask_previews/357bc801-89ff-476c-a49c-51147b21ebc8_large.jpg)
SOLVED: Calculate [H3O+] for each solution. there a different parts and values to this question. Each value is a part. pH = 8.35 to [H3O+] = ? M pH = 11.23 to [
The electrode potential of hydrogen electrode is when H3O+ ion concentration is 10^ 5 M (pH2 = 1atm) 1.) 0.059 V 2.) 0.295 V 3.) 0.118 V 4.) 0.59 V

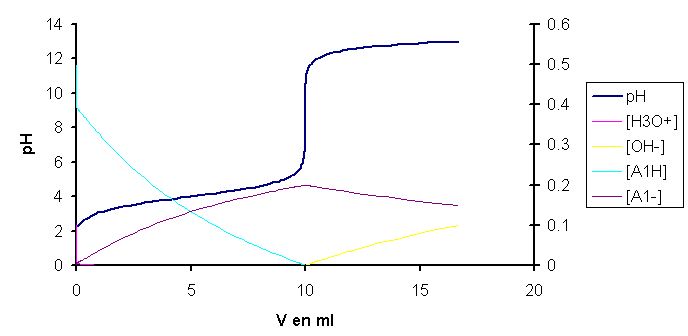
Observe the graph drawn between concentration of H3O+ Vs PH. Observe the graph carefully and answer the - Brainly.in
![SOLVED: What are [H3O+], [OH?], and pOH in a solution with a pH of 10.75? [ H3O+] = × 10 M [OH?] = × 10 M pOH = SOLVED: What are [H3O+], [OH?], and pOH in a solution with a pH of 10.75? [ H3O+] = × 10 M [OH?] = × 10 M pOH =](https://cdn.numerade.com/ask_previews/9bcb676e-2e4b-43ae-8a7f-0dd142f63174_large.jpg)
![What are the [H3O+] and the pH of a propanoic acid-propanoat | Quizlet What are the [H3O+] and the pH of a propanoic acid-propanoat | Quizlet](https://d2nchlq0f2u6vy.cloudfront.net/21/01/25/978cabc3ddc3d74d3e96a03212eb3a25/4ec214a0cf6c07a14220b9474a99d6f4/lateximg_large.png)


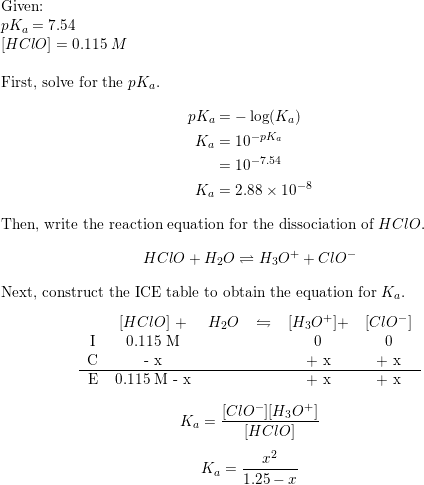


![What is the [OH^-] in a solution that has a [H_3O]=1.0*10^-6 M? | Socratic What is the [OH^-] in a solution that has a [H_3O]=1.0*10^-6 M? | Socratic](https://useruploads.socratic.org/vddVxwb9S2uSFVGMkcV6_kw-formula.jpg)


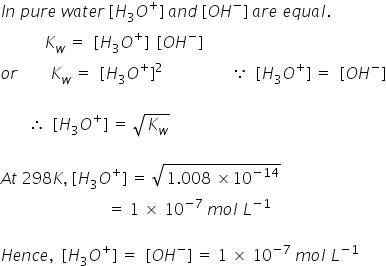

![SOLVED: What is the pH of a solution in which [H3O+] = 3.8 × 10-8 M? SOLVED: What is the pH of a solution in which [H3O+] = 3.8 × 10-8 M?](https://cdn.numerade.com/ask_previews/6d45f098-65ca-47ca-ad4a-bd8a807dcc71_large.jpg)
![Solved [H3O+] [OH-] pH pОН Acidic, Base or Neutral? | Chegg.com Solved [H3O+] [OH-] pH pОН Acidic, Base or Neutral? | Chegg.com](https://media.cheggcdn.com/media/f34/f34e841e-96b5-4eae-8369-3ddf8b4861c0/phpT8rw9L)
![Calculating [H₃O⁺] and pH (worked examples) (video) | Khan Academy Calculating [H₃O⁺] and pH (worked examples) (video) | Khan Academy](https://cdn.kastatic.org/ka_thumbnails_cache/e67920d2-30a0-40ef-bcfb-a7761c9673f6_1280_720_base.png)



![H3O+], [OH–], pH - BC Learning Network H3O+], [OH–], pH - BC Learning Network](https://s2.studylib.net/store/data/005610503_1-47ff8b2322e271c154ebb4db3f00887e-768x994.png)
![16.6: Finding the [H3O+] and pH of Strong and Weak Acid Solutions - Chemistry LibreTexts 16.6: Finding the [H3O+] and pH of Strong and Weak Acid Solutions - Chemistry LibreTexts](https://i.ytimg.com/vi/y7DTjgrcP-0/maxresdefault.jpg)
![pH, [H3O+], & [OH-] Calculations - YouTube pH, [H3O+], & [OH-] Calculations - YouTube](https://i.ytimg.com/vi/NlHDiLMEYuo/maxresdefault.jpg)