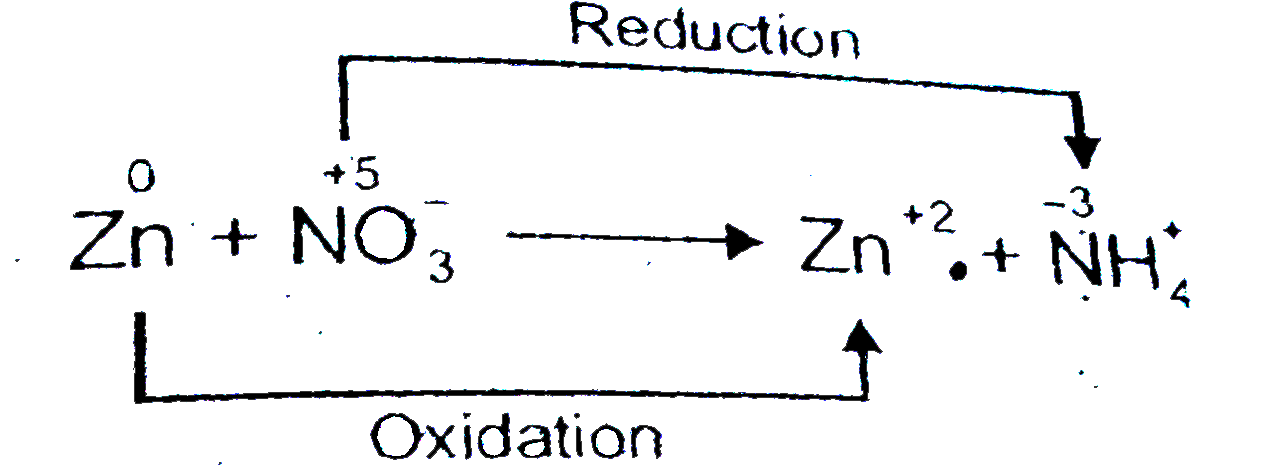
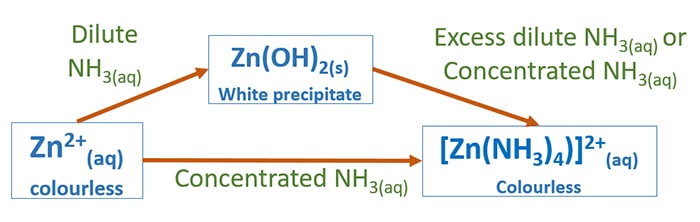
The Zinc Linchpin Motif in the DNA Repair Glycosylase MUTYH: Identifying the Zn2+ Ligands and Roles in Damage Recognition and Repair | Journal of the American Chemical Society

Aza-phenol Based Macrocyclic Probes Design for “CHEF-on” Multi Analytes Sensor: Crystal Structure Elucidation and Application in Biological Cell Imaging | ACS Omega
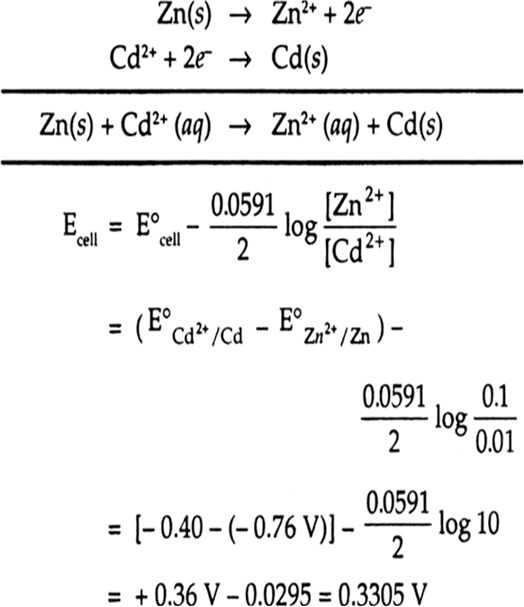
Calculate the emf of the cell Zn/Zn2+ (0.1 M) || Cd2+ (0.01 M) | Cd at 298 k. (given)E°Zn2+/Zn = – 0.76 V and E°Cd2+/Cd = – 0.40 V). from Chemistry Electrochemistry Class 12 CBSE

An Enzyme‐Activatable Engineered DNAzyme Sensor for Cell‐Selective Imaging of Metal Ions - Yi - 2021 - Angewandte Chemie International Edition - Wiley Online Library
47. If Zn2+/An electrode is diluted 1000 times then the change in electrode potential is 1. Increase of 29.5 mV 2. Increase of 59 mV 3. Decrease of 88.5 mV 4. Decrease of 91.1 mV.

Schematic Representation of the Mechanisms and Factors Driving Zn 2+... | Download Scientific Diagram

Measurement of Intracellular Free Zinc in Living Cortical Neurons: Routes of Entry | Journal of Neuroscience

Carrier-free nanoprodrug for p53-mutated tumor therapy via concurrent delivery of zinc-manganese dual ions and ROS - ScienceDirect

Halogenated Zn2+ Solvation Structure for Reversible Zn Metal Batteries | Journal of the American Chemical Society

Schematic Representation of the Cellular Zinc Pool. Gray arrows and... | Download Scientific Diagram
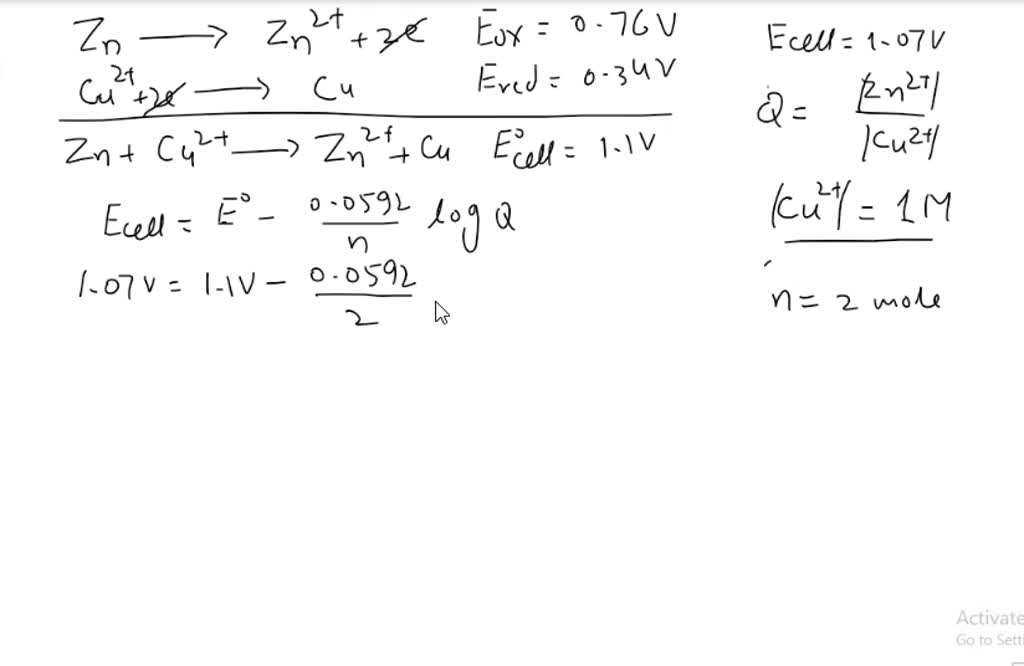
SOLVED: What is the Nickel (II)-ion concentration in the electrochemical cell if the cell potential is 0.34 V at 25°C? Zn(s) | Zn2+(1.00 M) || Ni2+(aq) | Ni(s

Xitoy ion almashinadigan qatronlar onlayn xelatli qatronlar yetkazib beruvchilar, ishlab chiqaruvchilar, zavod - moslashtirilgan ion almashinadigan qatronlar onlayn ulgurji savdosi - Lanlang

Consider the following galvanic cell: What happens to E as the concentration of Zn^2+ is increased? as the concentration of Ag+ is increased? What happens to E^0 in these cases? | Homework.Study.com

Zn2+ Blocks Annealing of Complementary Single-Stranded DNA in a Sequence-Selective Manner | Scientific Reports
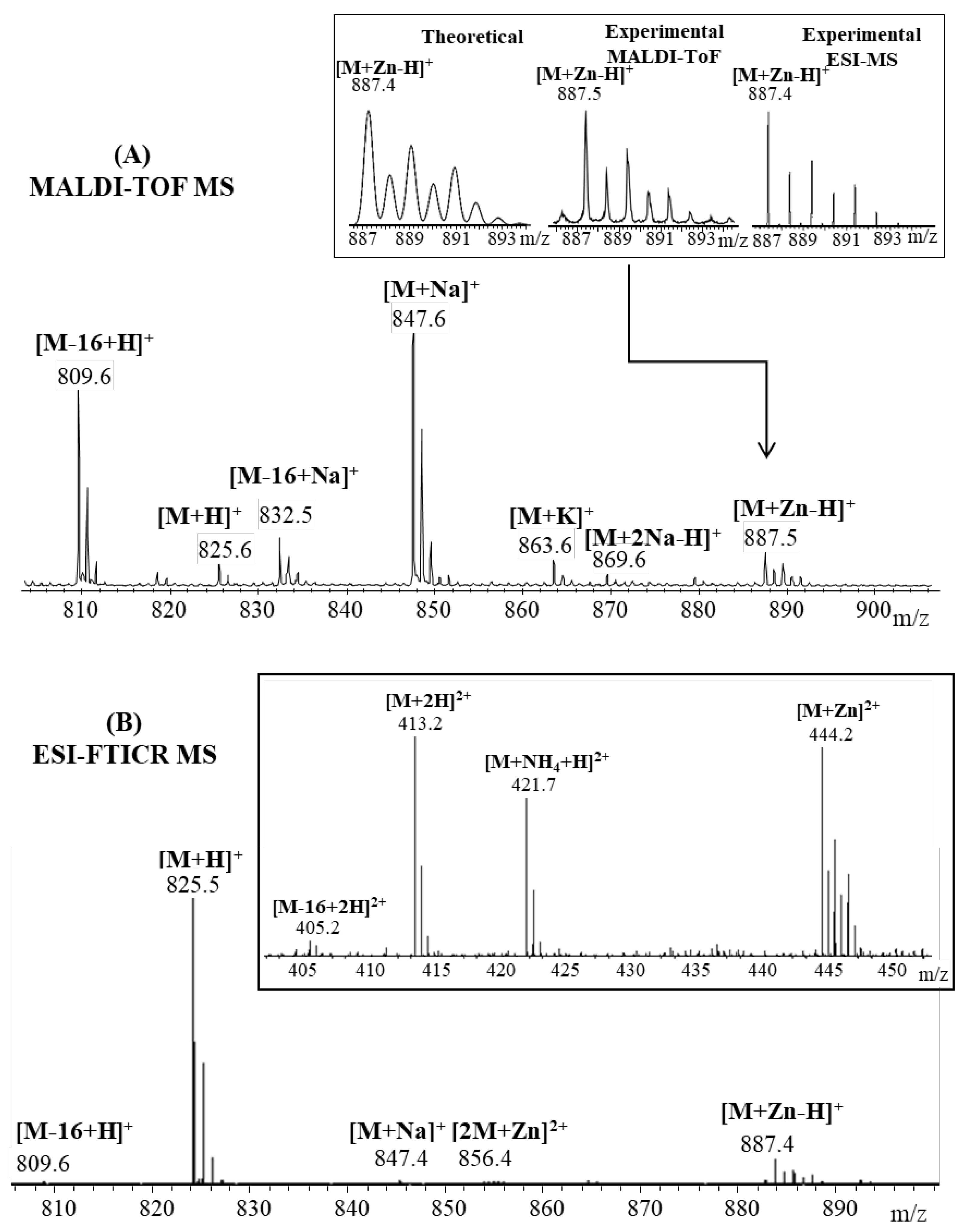
Pharmaceuticals | Free Full-Text | Cu and Zn Interactions with Peptides Revealed by High-Resolution Mass Spectrometry

SOLVED: Please help solve #18 #19: Part D-Dissolving Insoluble Solids Equilibrium system: Zn(OH)2(s) 5 Zn2+(aq) + 2 OH-(aq) Kse<<1 Observations 17. Step 1 Adding1 drop of NaOHagto 7n(NO2ag Cloudy white Step 2