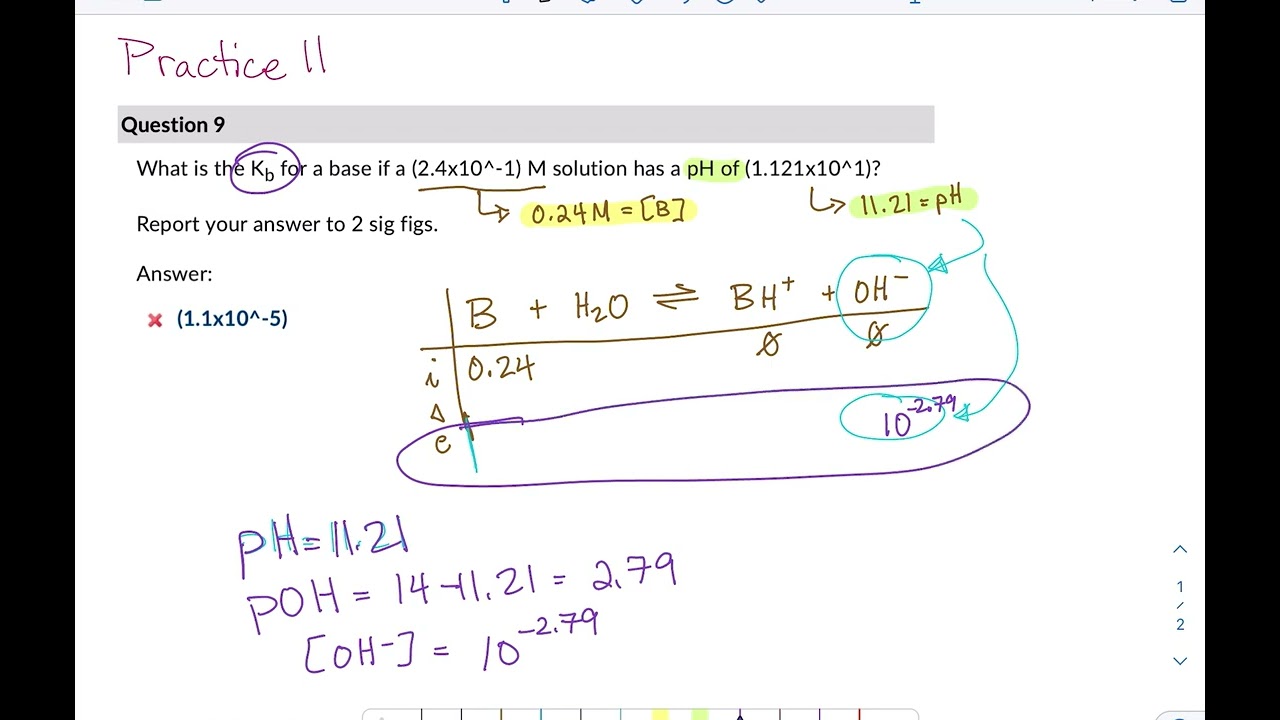
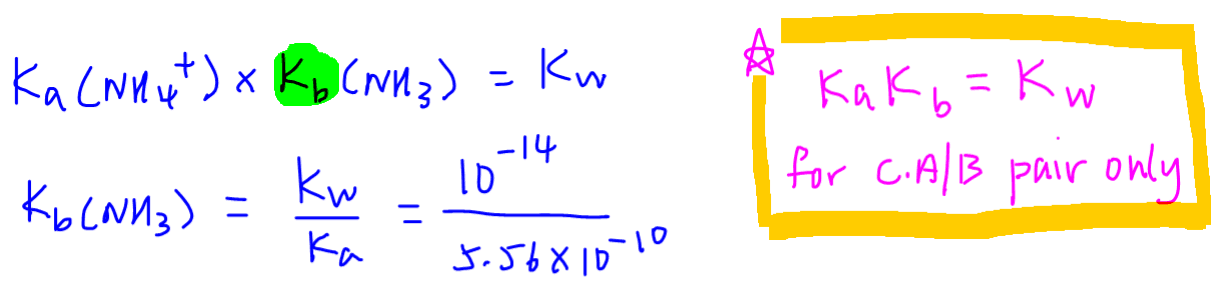What is the pH of a 0.402 M aqueous solution of NaCH3COO? Ka (CH3COOH) = 1.8x10-5 | Homework.Study.com

Calculate the pH of a 0.10 M solution of hydrazine, N2H4. Kb for hydrazine is 1.3×10−6 - Home Work Help - Learn CBSE Forum
Calculate pH of the following mixtures. Given that Ka = 1.8 × 10^–5 and Kb = 1.8 × 10^–5: - Sarthaks eConnect | Largest Online Education Community

The pH profiles for the V 1 /K B function with neutral substrates. (A)... | Download Scientific Diagram


![Calculating [OH-], pH and pOH from Kb Calculating [OH-], pH and pOH from Kb](https://www.mi.mun.ca/users/pfisher/chemistry1011_135/img007.gif)