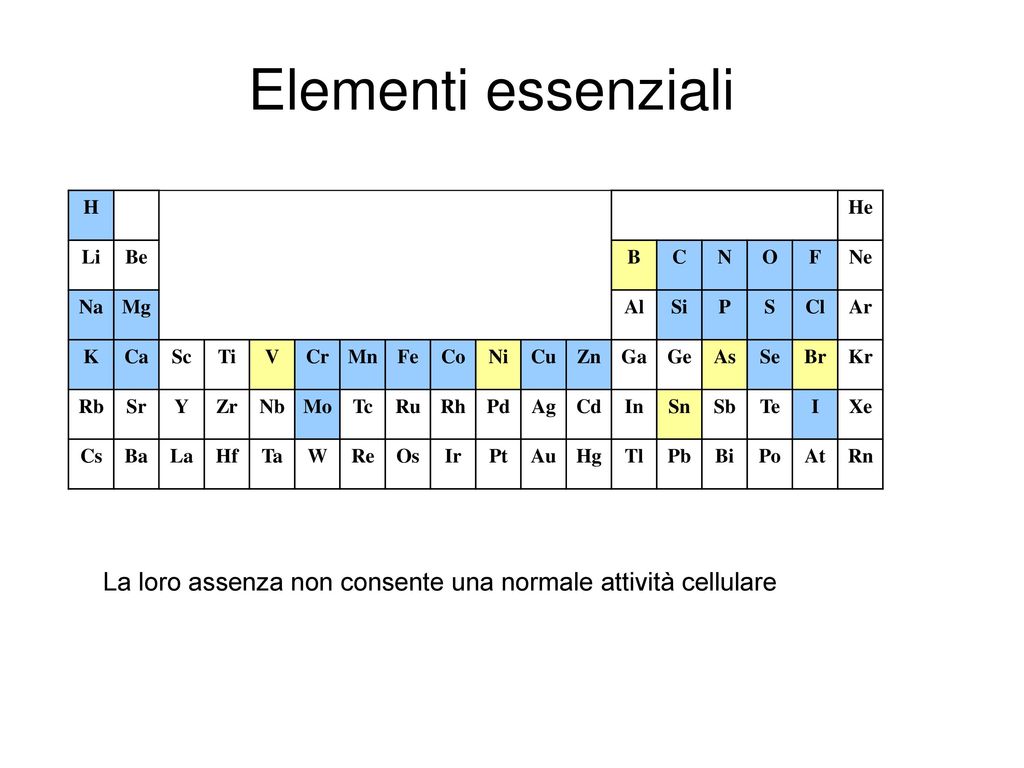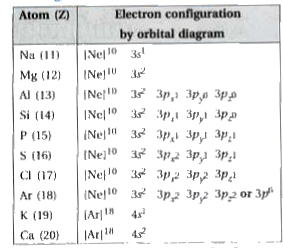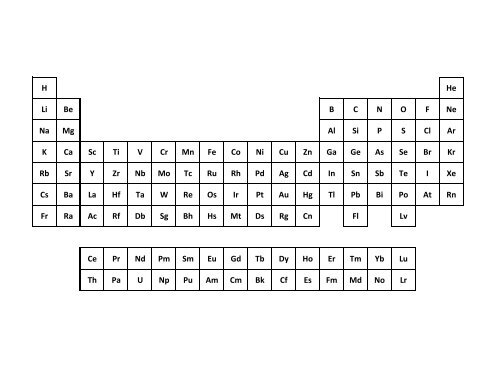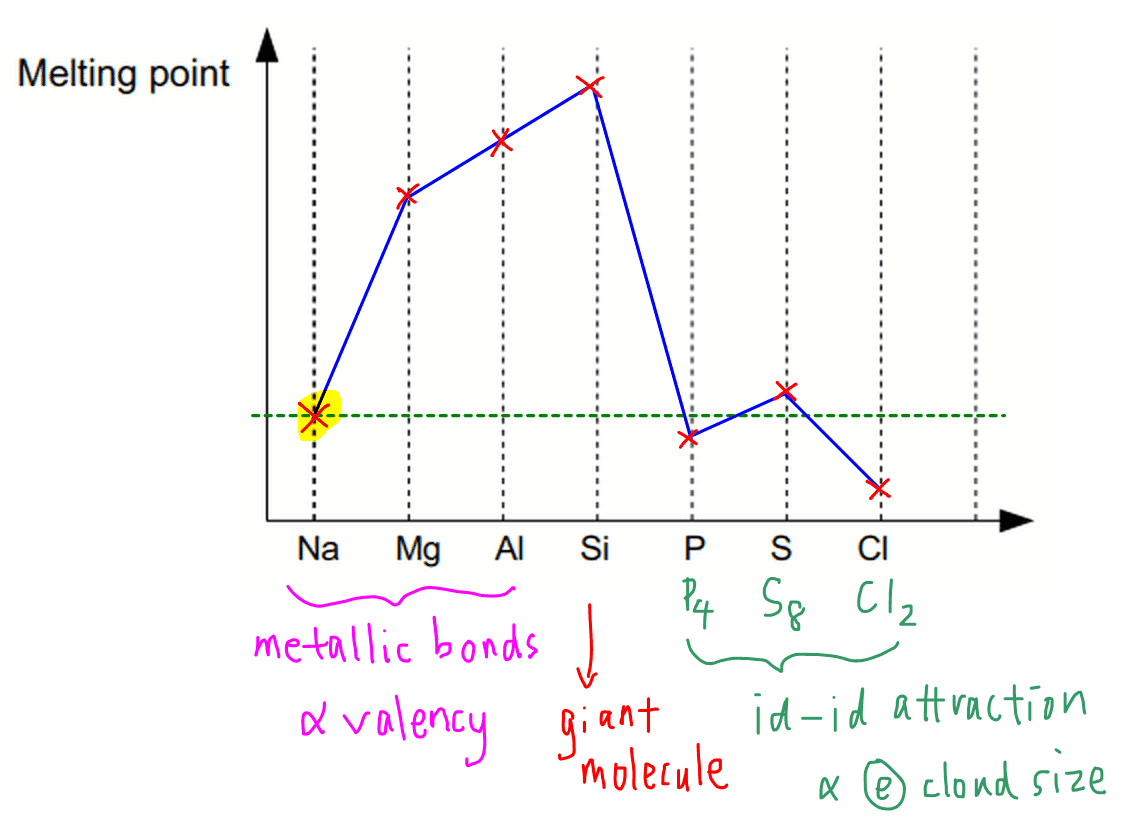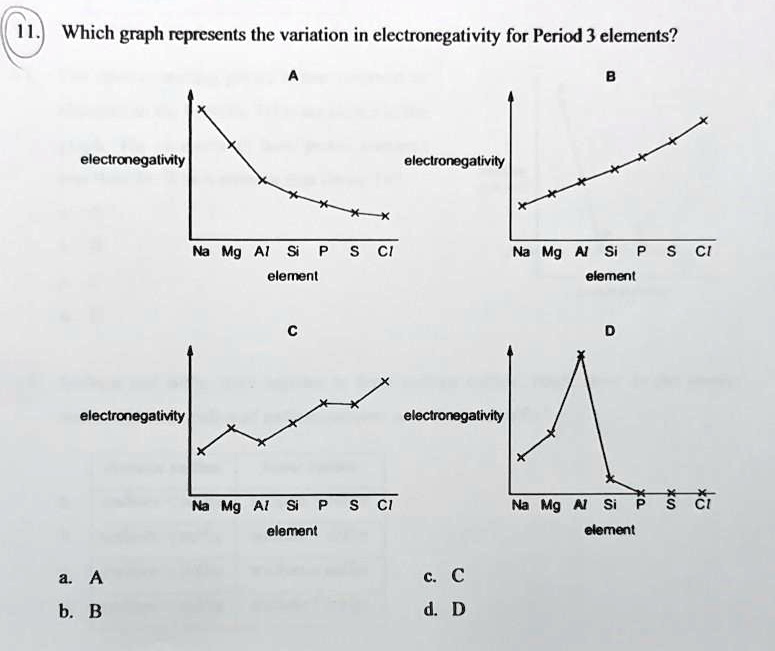
SOLVED: Which graph represents the variation in electronegativity for Period 3 elements? electronegalivty electronegalivity Ma Mg Al elemenl Na Mg N Si elemenl CI electronegativty electronagativity Na Mg Al Si elemant Mg

Which one of the following is correct order of second ionisation potential of `Na, Ne Mg` and `Al`? - YouTube
Na, Mg, and Al are 3 elements of the 3rd period in the modern periodic table, having group number 1, 2, and 13 respectively. Which one of these elements has the maximum
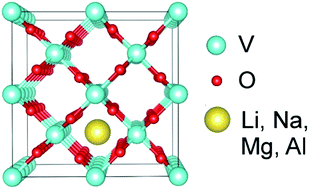
Comparison of Li, Na, Mg and Al-ion insertion in vanadium pentoxides and vanadium dioxides - RSC Advances (RSC Publishing)



![Chemistry] Topic Checklist: Metals and the Reactivity Series Flashcards | Quizlet Chemistry] Topic Checklist: Metals and the Reactivity Series Flashcards | Quizlet](https://o.quizlet.com/yDX1KeN3mt0M8PKXbCGMMQ.png)