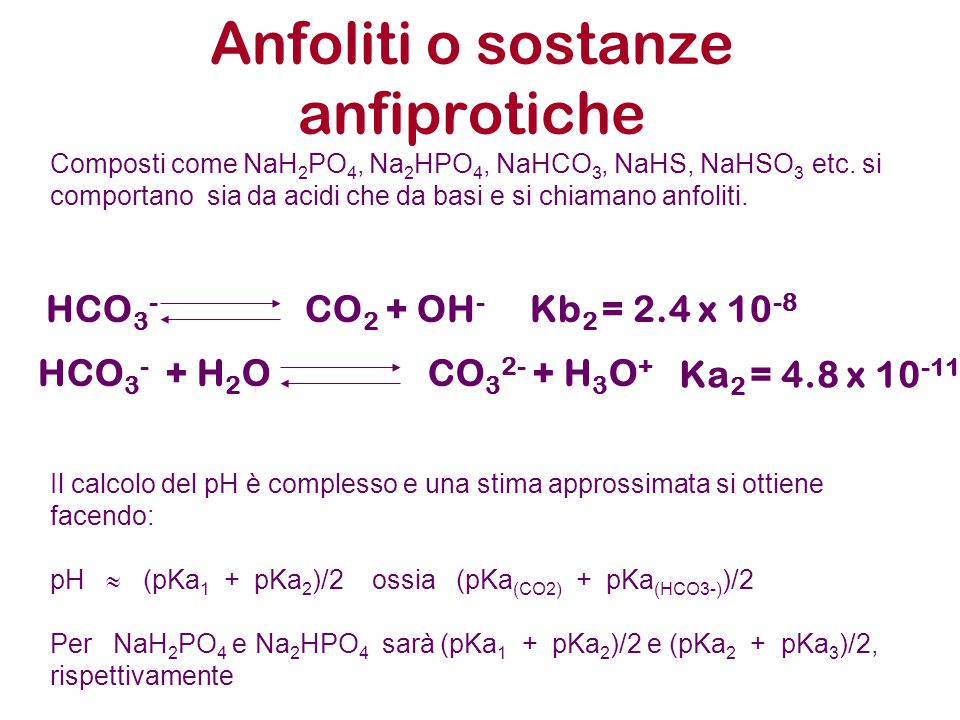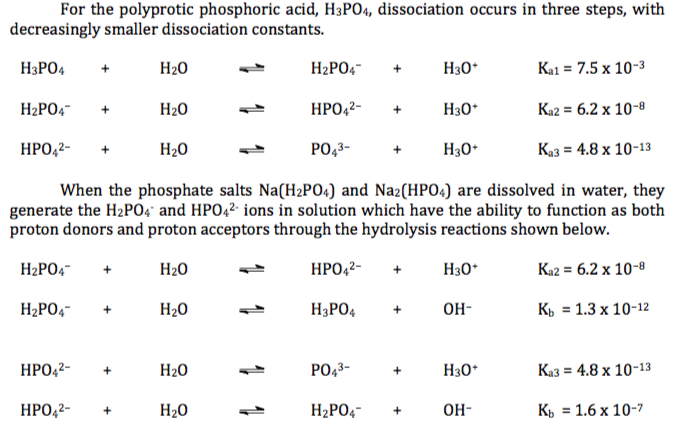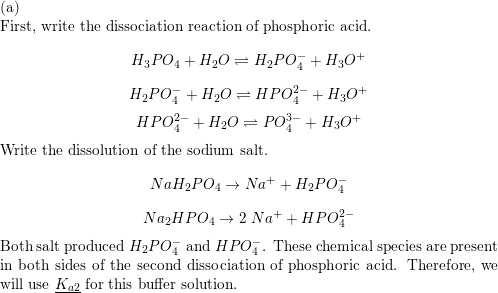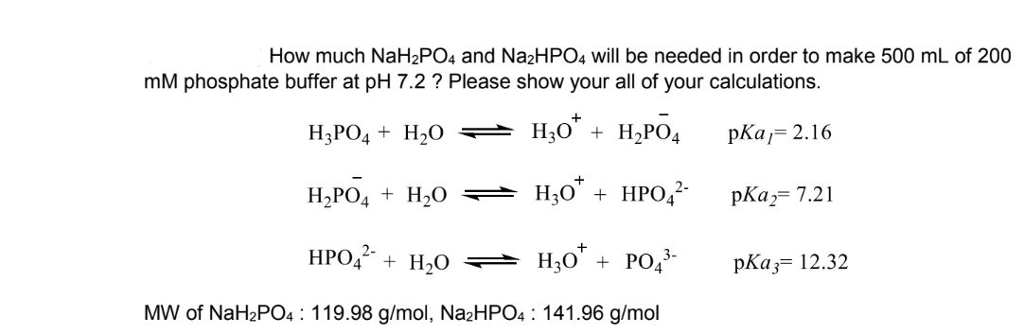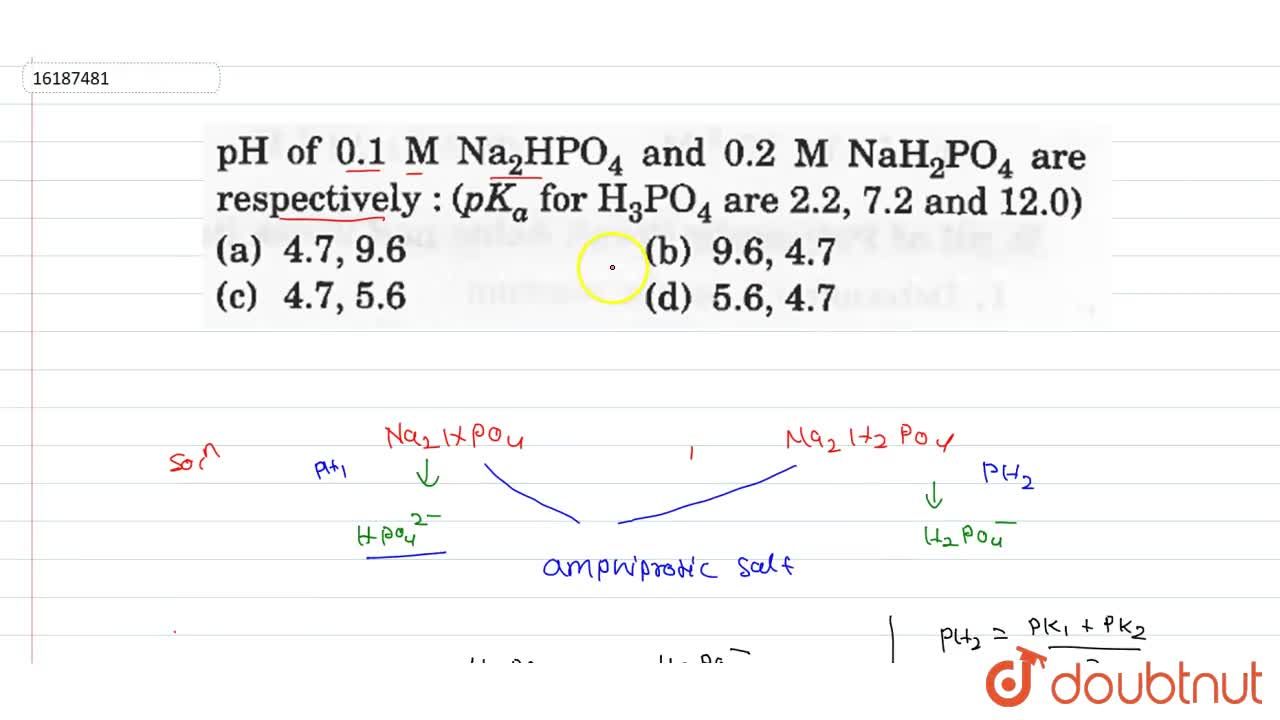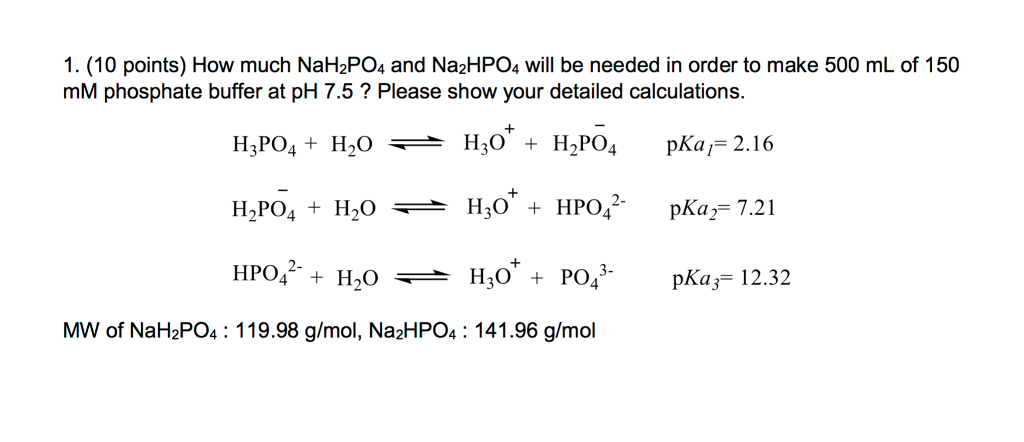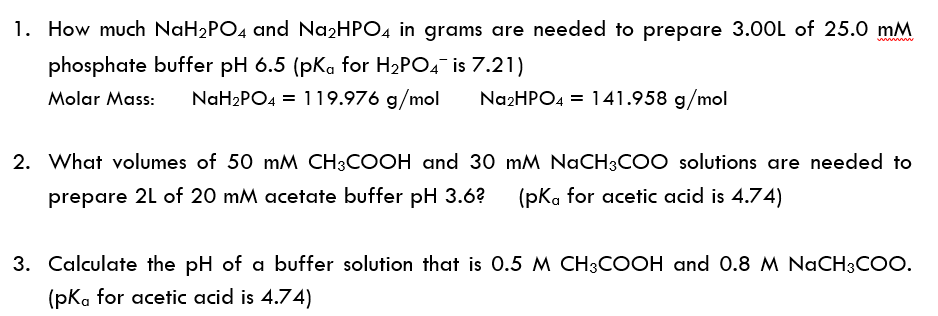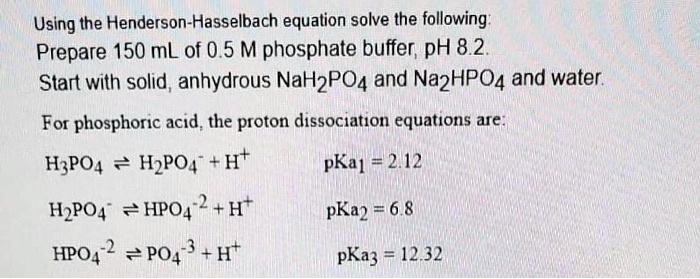
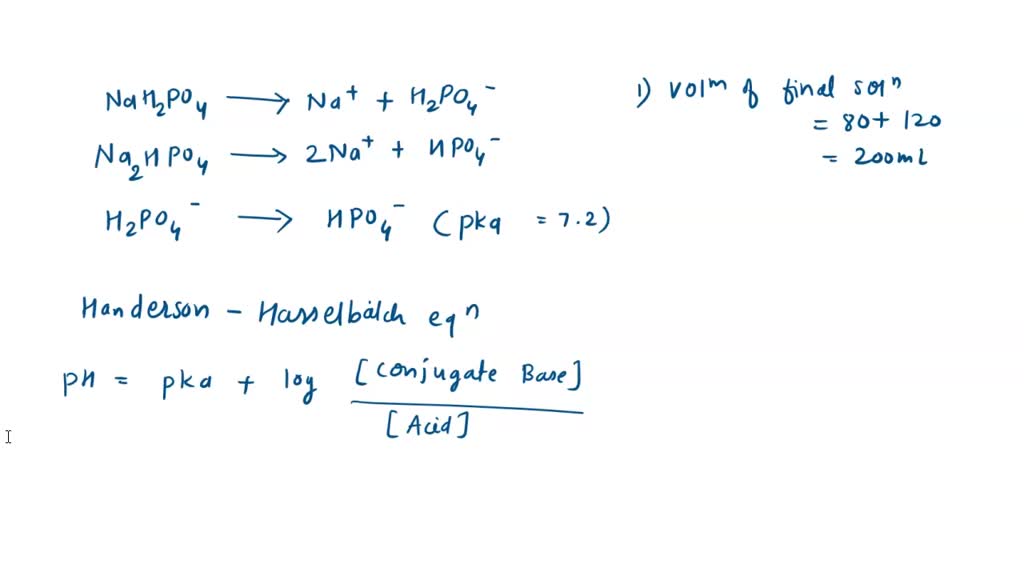
SOLVED: Using the Henderson-Hasselbach equation solve the following: Prepare 150 mL of 0.5 M phosphate buffer; pH 8.2 Start with solid, anhydrous NaHzPO4 and NazHPO4 and water: For phosphoric acid; the proton

The experiments were performed in 1 ml of 20 mM Na2HPO4-NaH2PO4 buffer... | Download Scientific Diagram

The typical titration curve of the Na2HPO4/NaH2PO4 solution obtained as... | Download Scientific Diagram
Potential response characteristics in 0.1 M Na2HPO4 solution at pH 9.0... | Download Scientific Diagram
![PBST [10X]; Phosphate buffered saline with Tween-20; 80mM Na2HPO4, 1.5M NaCl, 20mM KH2PO4, 30mM KCl, 0.5% Tween-20, pH 7.4 - Cepham Life Sciences Research Products PBST [10X]; Phosphate buffered saline with Tween-20; 80mM Na2HPO4, 1.5M NaCl, 20mM KH2PO4, 30mM KCl, 0.5% Tween-20, pH 7.4 - Cepham Life Sciences Research Products](https://www.cephamls.com/wp-content/uploads/2019/02/10385-1.jpg)
PBST [10X]; Phosphate buffered saline with Tween-20; 80mM Na2HPO4, 1.5M NaCl, 20mM KH2PO4, 30mM KCl, 0.5% Tween-20, pH 7.4 - Cepham Life Sciences Research Products

Table 2 from Dynamic approach to predict pH profiles of biologically relevant buffers | Semantic Scholar

If 2.5 moles each of H3PO4,NaH2PO4,Na2HPO4 and Na3PO4 are mixed together to form an aqueous solution, then the resulting pH is:Given values of Ka are: Ka1 = 10^-3 Ka2 = 10^-7 Ka3 = 10^-13

Calculate the pH of a buffer solution obtained by dissolving 25.0 g of KH2PO4(s) and 38.0 g of Na2HPO4(s) in water and then diluting to 1.00 L. | Homework.Study.com




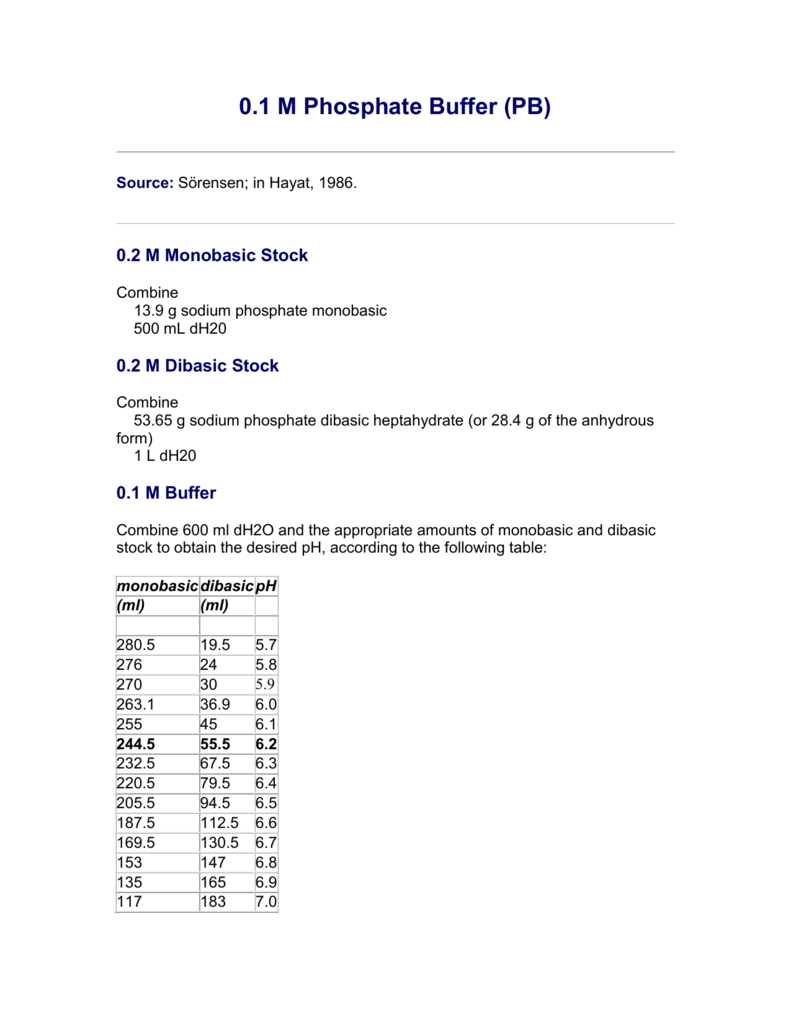


![BS020a] 1M Sodium Phosphate, pH x.x | Biosolution BS020a] 1M Sodium Phosphate, pH x.x | Biosolution](http://biosolution.cafe24.com/wp-content/uploads/2017/11/BS020-Sodium-Phosphate-Buffer.jpg)