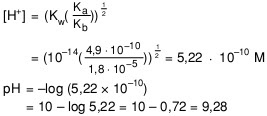pH of 0.1 M solution of NH_4CN solution is x. If its concentration is increased to 0.2 M then pH of the solution will be (1) 2x (2) x/2 (3) x (4) x/4
PPT - All cations except act as ______ in aqueous solutions, e.g., PowerPoint Presentation - ID:5168415

Calculate the pH of 0.01 M solution of NH4CN . The dissociation constants Ka for HCN = 6.2 × 10^-10 and Kb for NH3 = 1.6 × 10^-5 .

Tentukan ph larutan 0.01 M NH4CN apabila Ka HCN=5×10 pngkat min 10 Kb NH4OH=1.8×10pngkt min 5?? - Brainly.co.id

SOLVED:Calculate the pH of a solution prepared from 0.200 mol of NH4CN and enough water to make 1.00 L of solution.

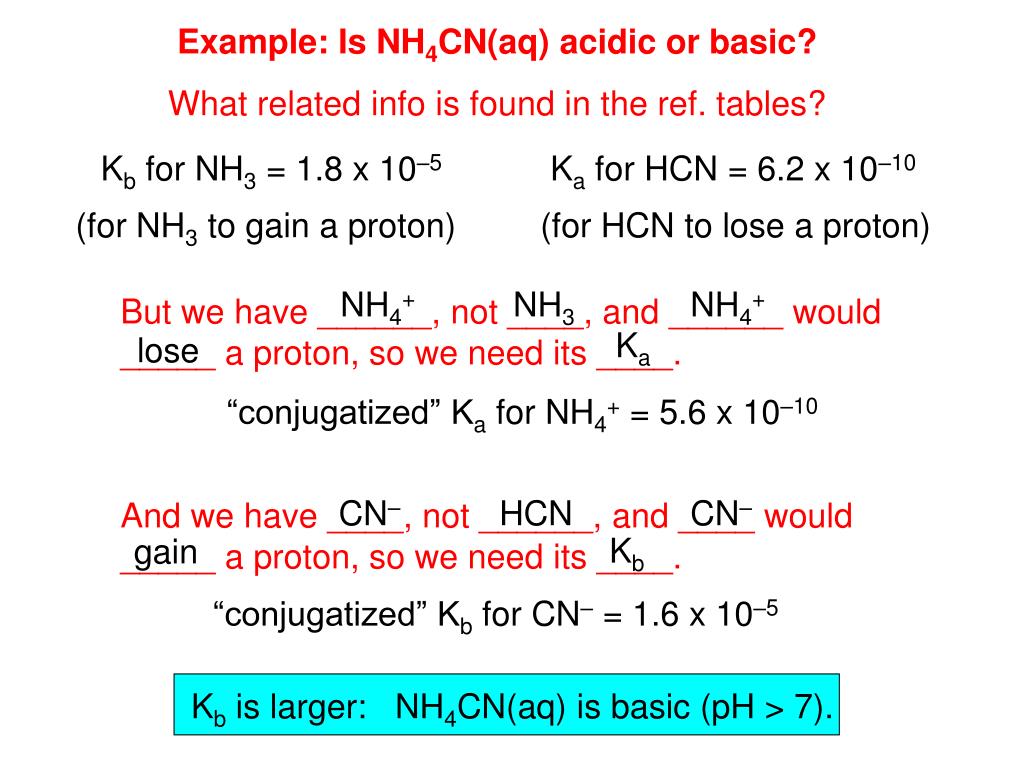
OneClass: The Ka of NH4+ is 5.6 × 10âˆ'10. The Kb of CNâˆ' is 2 × 10âˆ'5. The pH of a salt solution...

Polymers | Free Full-Text | Tuning the Morphology in the Nanoscale of NH4CN Polymers Synthesized by Microwave Radiation: A Comparative Study

Calculate the pH of 0.01 M solution of NH4CN . The dissociation constants Ka for HCN = 6.2 × 10^-10 and Kb for NH3 = 1.6 × 10^-5 .

calculate the degree of hydrolysis and pH of NH4CN at 298 degree centigrade given K A equals to 4 99 - Chemistry - Equilibrium - 11926887 | Meritnation.com
![Calculate the degree of hydrolysis and pH of 0.02M ammonium cyanide (NH4CN) at 298 K. [K1 of HCN = 4.99 × 10^-9, Kb for NH4OH = 1.77 × 10^-5] Calculate the degree of hydrolysis and pH of 0.02M ammonium cyanide (NH4CN) at 298 K. [K1 of HCN = 4.99 × 10^-9, Kb for NH4OH = 1.77 × 10^-5]](https://i.ytimg.com/vi/aiZVXDgX23w/maxresdefault.jpg)