![SOLVED: Calculate the pH of each solution given the following [H3O+] or [OH−] values. H3O+] = 9.0×10−4 M Express your answer using two decimal places. [H3O+]= 1.0×10−8 M Express your answer using SOLVED: Calculate the pH of each solution given the following [H3O+] or [OH−] values. H3O+] = 9.0×10−4 M Express your answer using two decimal places. [H3O+]= 1.0×10−8 M Express your answer using](https://cdn.numerade.com/ask_previews/7607decf-a386-4944-83d1-a7bcced790cf_large.jpg)
SOLVED: Calculate the pH of each solution given the following [H3O+] or [OH−] values. H3O+] = 9.0×10−4 M Express your answer using two decimal places. [H3O+]= 1.0×10−8 M Express your answer using
![SOLVED: Be sure to answer all parts. The optimum pH of a swimming pool is 7.35. Calculate the value of [H3O*] and [OH] at this pH [H3O*] x 10 (select) W [OH]= SOLVED: Be sure to answer all parts. The optimum pH of a swimming pool is 7.35. Calculate the value of [H3O*] and [OH] at this pH [H3O*] x 10 (select) W [OH]=](https://cdn.numerade.com/ask_previews/28e6f787-6e7d-432d-894e-c738278418d3_large.jpg)
SOLVED: Be sure to answer all parts. The optimum pH of a swimming pool is 7.35. Calculate the value of [H3O*] and [OH] at this pH [H3O*] x 10 (select) W [OH]=
![SOLVED: Calculate [H3O+] for each solution. there a different parts and values to this question. Each value is a part. pH = 8.35 to [H3O+] = ? M pH = 11.23 to [ SOLVED: Calculate [H3O+] for each solution. there a different parts and values to this question. Each value is a part. pH = 8.35 to [H3O+] = ? M pH = 11.23 to [](https://cdn.numerade.com/ask_previews/357bc801-89ff-476c-a49c-51147b21ebc8_large.jpg)
![Calculating [H3O+] from pH - YouTube Calculating [H3O+] from pH - YouTube](https://i.ytimg.com/vi/TOIeMRuRU08/hqdefault.jpg)


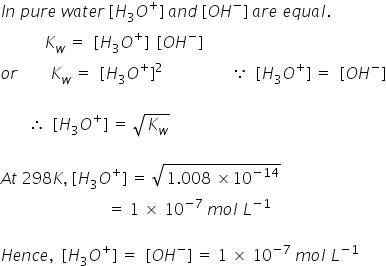




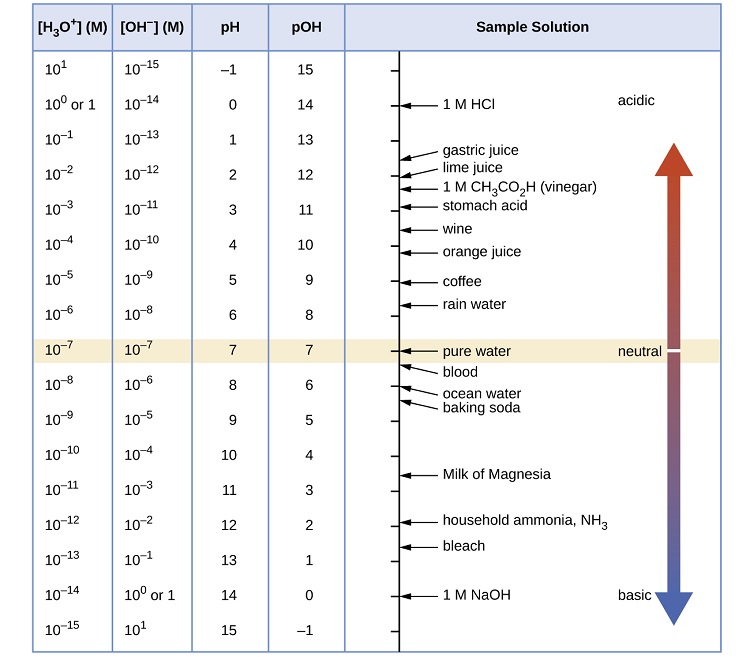


![Answered: Determine the pH for [H3O*]=1.8 × 10-4… | bartleby Answered: Determine the pH for [H3O*]=1.8 × 10-4… | bartleby](https://content.bartleby.com/qna-images/question/5291ca13-f244-465e-944a-4b98550ee0d6/230f2f39-6ed0-40fb-843a-80af9cf2c8cc/iftw73e_processed.png)
.PNG)




![Calculating [H₃O⁺] and pH (worked examples) (video) | Khan Academy Calculating [H₃O⁺] and pH (worked examples) (video) | Khan Academy](https://cdn.kastatic.org/ka_thumbnails_cache/e67920d2-30a0-40ef-bcfb-a7761c9673f6_1280_720_base.png)