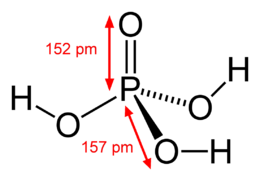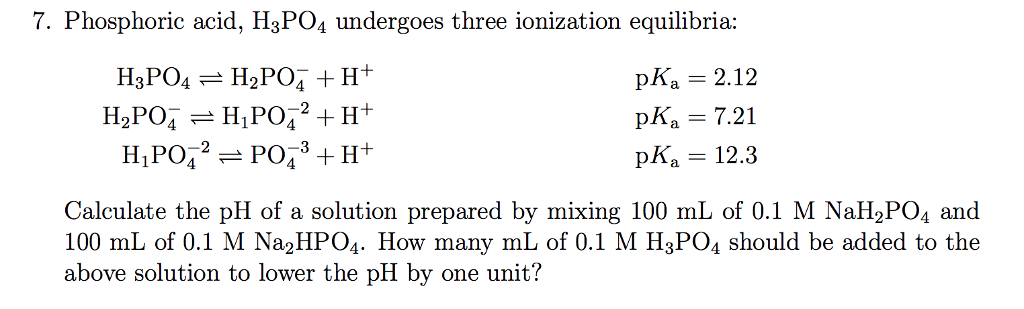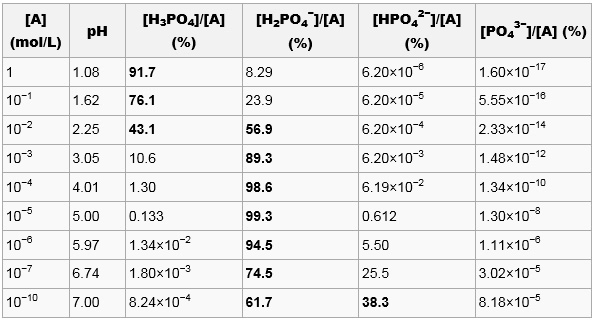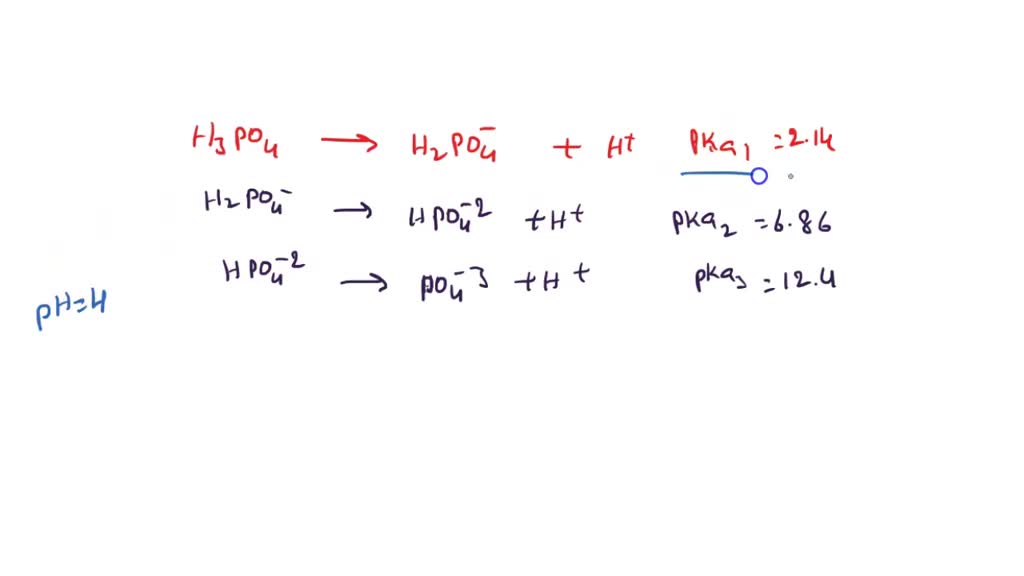
SOLVED: Phosphoric acid (H3PO4) has three pKa values: 2.14, 6.86, and 12.4. Write the chemical formula for the major form of this molecule in an aqueous solution at pH = 4. This

SOLVED: Calculate the pH of a 5.0 M phosphoric acid (HzPO4) solution and the equilibrium concentrations of all the other species in the solution (HzPO4, HzPOA HPOA 2 and POA 3 7,
![In a solution 0.1 M H3PO4 acid, concentration of H^+ is :[Use : Ka1 = 10^-3, Ka2 = 10^-7, Ka3 = 10^-12 ] In a solution 0.1 M H3PO4 acid, concentration of H^+ is :[Use : Ka1 = 10^-3, Ka2 = 10^-7, Ka3 = 10^-12 ]](https://dwes9vv9u0550.cloudfront.net/images/10282364/81604d52-36ea-4174-8f80-9b1dc198cc50.jpg)
In a solution 0.1 M H3PO4 acid, concentration of H^+ is :[Use : Ka1 = 10^-3, Ka2 = 10^-7, Ka3 = 10^-12 ]

TRACCIA 9 Calcolare il pH di una soluzione ottenuta sciogliendo 12 g di NaH2PO4 in 100 ml di KOH 2 M. (H3PO4: Ka1=7,6x10-3, Ka2=6,2x10-8, Ka1=4,4x10-13). - ppt scaricare
![pH of 0.1 M H3PO4 acid solution is :[For the given acid: Ka1 = 10^-3, Ka2 = 10^-7 and Ka3 = 10^-12 ] pH of 0.1 M H3PO4 acid solution is :[For the given acid: Ka1 = 10^-3, Ka2 = 10^-7 and Ka3 = 10^-12 ]](https://haygot.s3.amazonaws.com/questions/1842609_1287746_ans_4acafe7dff4645fda56556fe5ef9778f.jpg)
pH of 0.1 M H3PO4 acid solution is :[For the given acid: Ka1 = 10^-3, Ka2 = 10^-7 and Ka3 = 10^-12 ]

PH finale di soluzione di H3PO4 a cui si aggiunge Na OH - 19/2/2020 PH finale di soluzione di H3PO4 - Studocu

Variation of pH and EC in HCl-4-HU, H2SO4-4-HU, and H3PO4-4-HU with... | Download Scientific Diagram
![pH of 0.1 M H3PO4 acid solution is :[For the given acid: Ka1 = 10^-3, Ka2 = 10^-7 and Ka3 = 10^-12 ] pH of 0.1 M H3PO4 acid solution is :[For the given acid: Ka1 = 10^-3, Ka2 = 10^-7 and Ka3 = 10^-12 ]](https://haygot.s3.amazonaws.com/questions/1844080_1287766_ans_5cdaae94561f4a2599341308914a04c0.jpg)
![Change of phosphate species with pH in H3PO4 [5]. | Download Scientific Diagram Change of phosphate species with pH in H3PO4 [5]. | Download Scientific Diagram](https://www.researchgate.net/publication/261995856/figure/fig10/AS:269753218498564@1441325587360/Change-of-phosphate-species-with-pH-in-H3PO4-5.png)