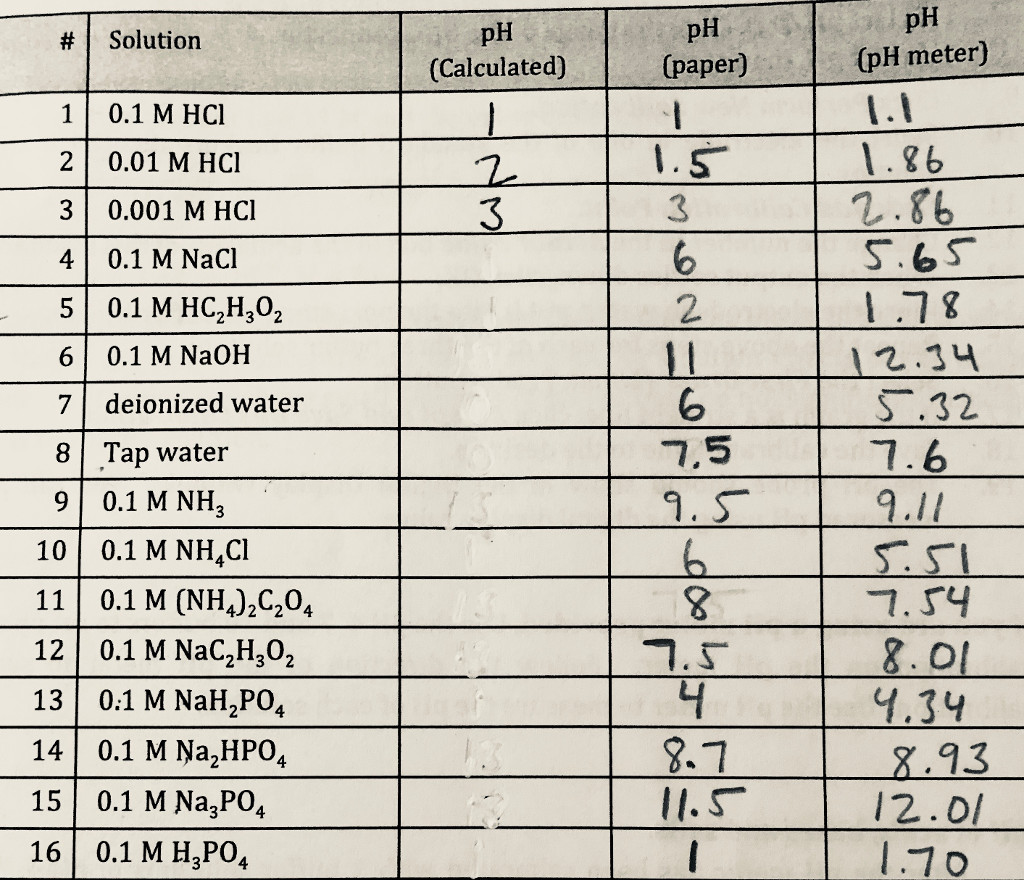
Effect of concentration (a) Tris-HCl buffer pH 6.5 at dilution of urea... | Download Scientific Diagram

A solution contains 0.09 M HCI , 0.09 M CHCl2COOH , and 0 .1 M CH3COOH . The pH of this solution is 1 . K for CHCl2COOH is 1.25 × 10^-x . The value of x is :[Given : Ka for CH3COOH = 10^-5 )
SOLVED: 'What are the pH of these solutions? 0.1 M HCI 0.001 M HCI 0.00001 M HCI Distilled Water 0.00001 M NaOH 0.001 M NaOH 0.1 M NaOH'
Calculate pH for : (a) 0.001 N NaOH, (b) 0.01 N Ca(OH)2 - Sarthaks eConnect | Largest Online Education Community