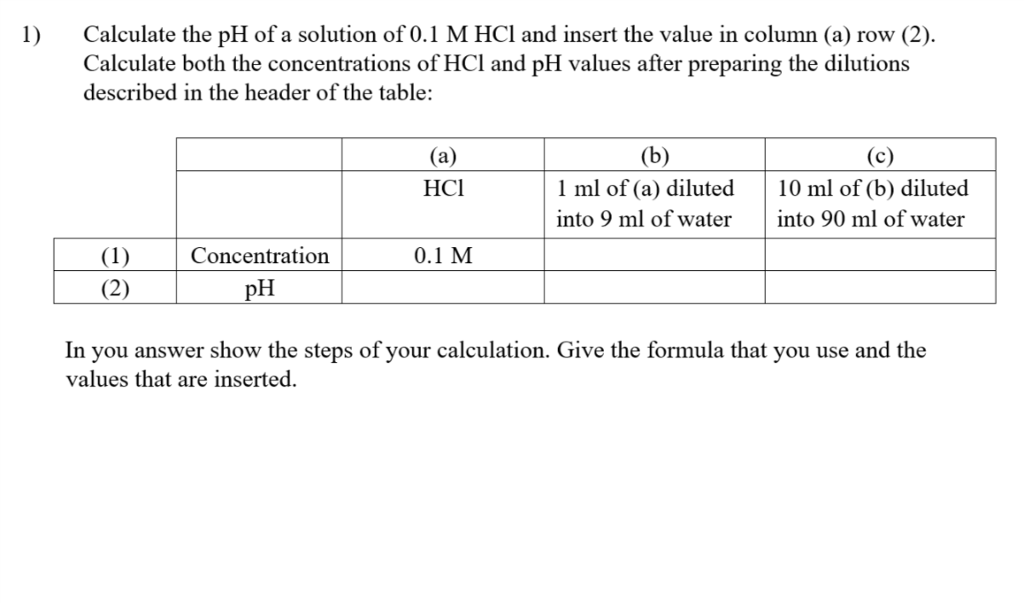
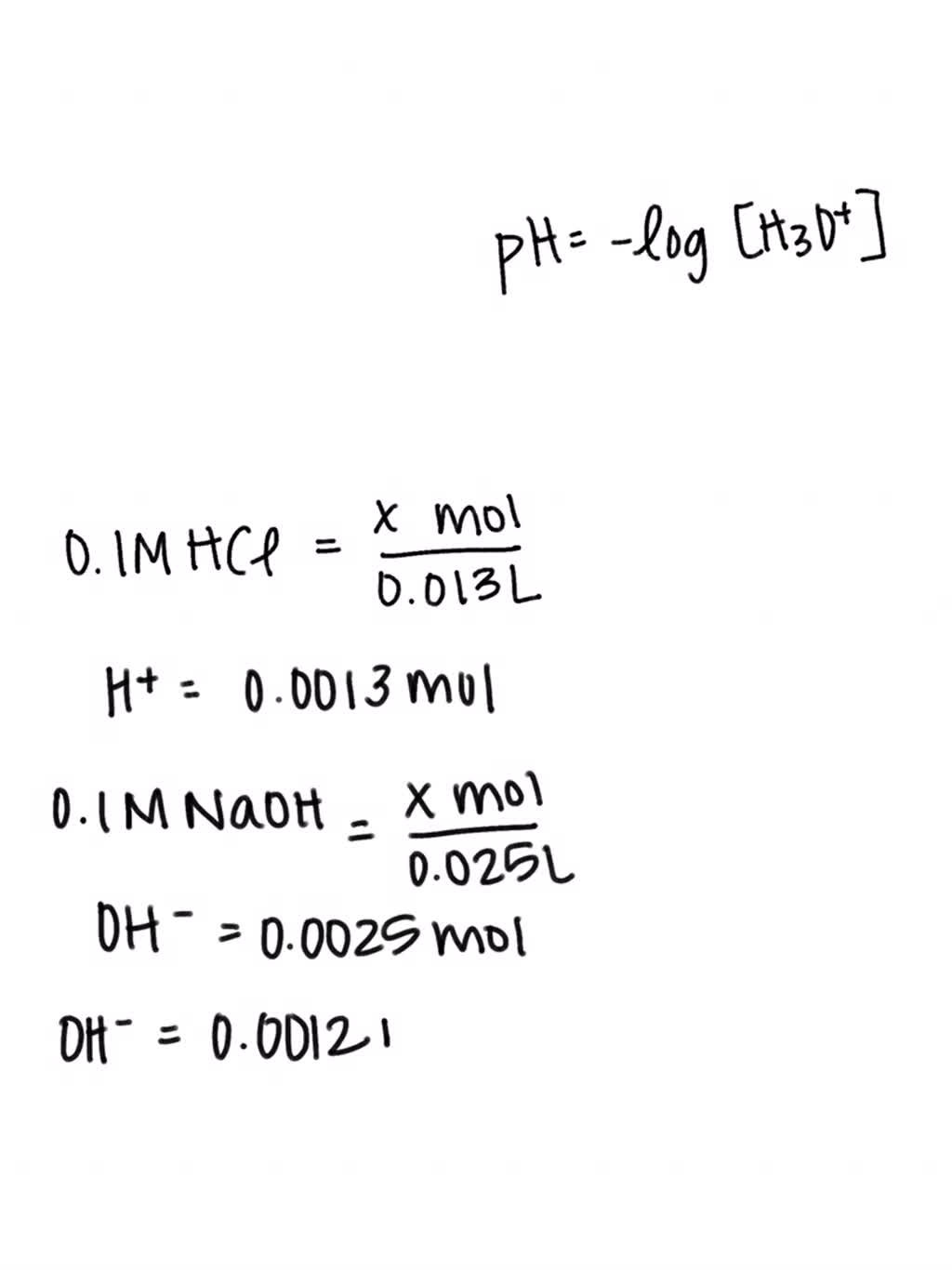
To a 50 ml of 0.1 M HCl solution , 10 ml of 0.1 M NaOH is added and the resulting solution is diluted to 100 ml. What is change in pH of the HCl solution ?

Calculate the ph of a solution formed by mixing 100 ml of 0.1m hcl and 9.9ml of 1m naoh - Brainly.in

SOLVED: 1. Calculate the pH if you added 3 mL of 0.1 M HCL to a) 97 mL of pure water at pH 7, and b) 100 mL of phosphate buffer (0.063

What is the pH of solution in which `25.0` mL of `0.1` M NaOH is added to 25 mL of `0.08`M HCl a... - YouTube

Hydrochloric acid solution, Volumetric, Reag. Ph. Eur., 0.1 M HCl (0.1N), Honeywell Fluka | Fisher Scientific