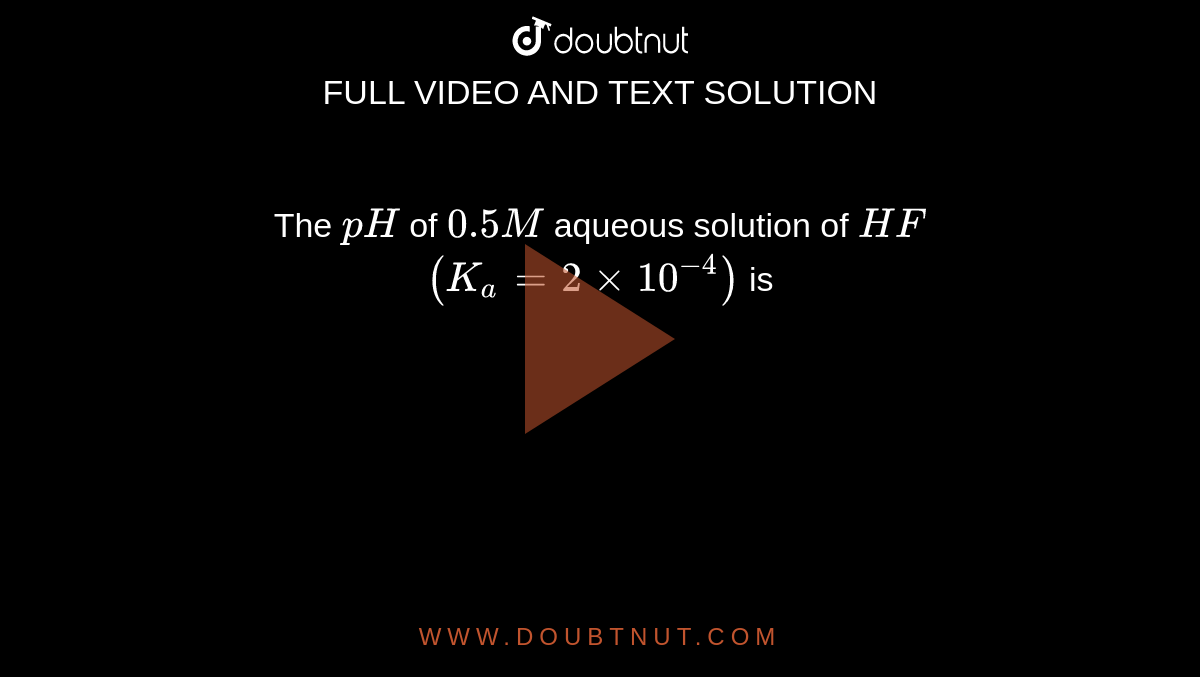
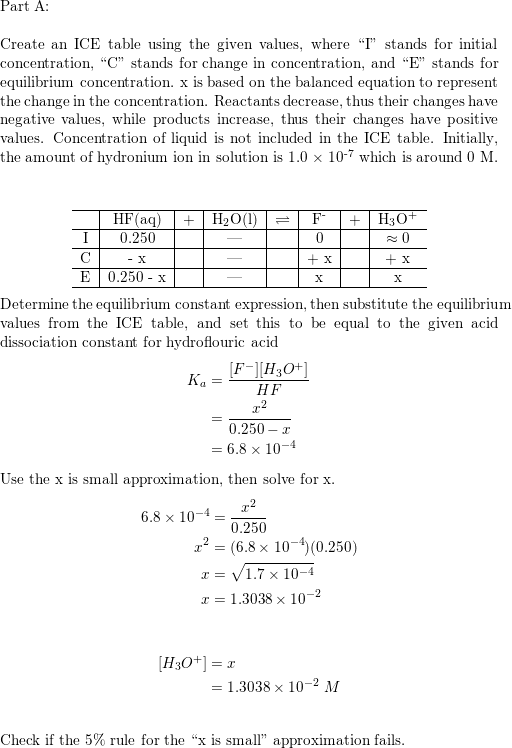
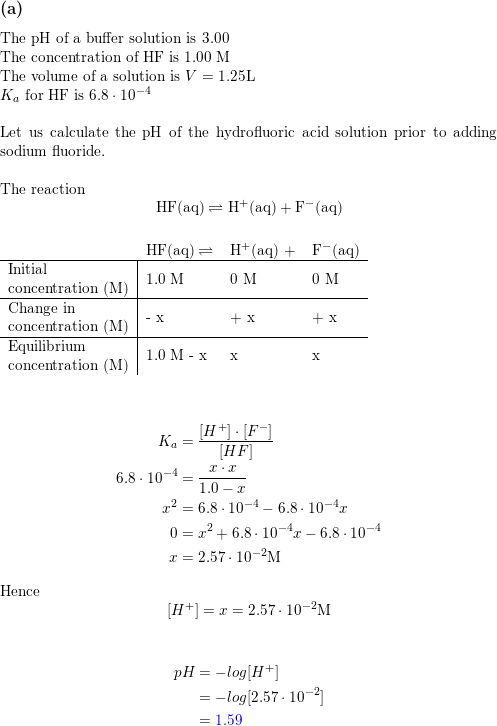
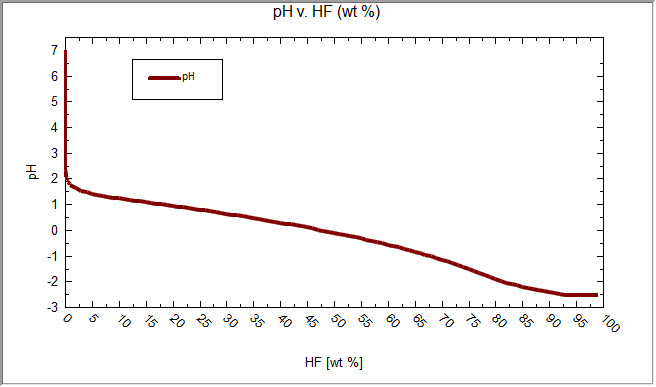
Determine the pH of HF solution of each of the following concentrations - Home Work Help - Learn CBSE Forum

SOLVED: Given that pKa = 3.18 at 25 %C for HF, what is the pH of 0.155 mol L-1 NaF(aq) at 25 *C? What is the equilibrium concentration of HF? pH 8.2 (

A 50.00ml sample of 0.200M hydrofluoric acid (HF) is titrated with 0.200M NaOH. The Pka of HF is 3.452. a) Calculate the pH of the HF solution before titration. b) Calculate the
✓ Solved: Calculate the pH of a solution that contains 1.0 M HF and 1.0 M HOC 6 H 5 . Also calculate...

SOLVED: 'Part A If a solution of HF (Ka = 6.8 x 10 has a pH of 3.30 , calculate the concentration of hydrofluoric acid Express your answer using two significant figures

The ionization constant of HF is 3.2 X 10-4. Calculate the degree of dissociation of HF in its..... - YouTube