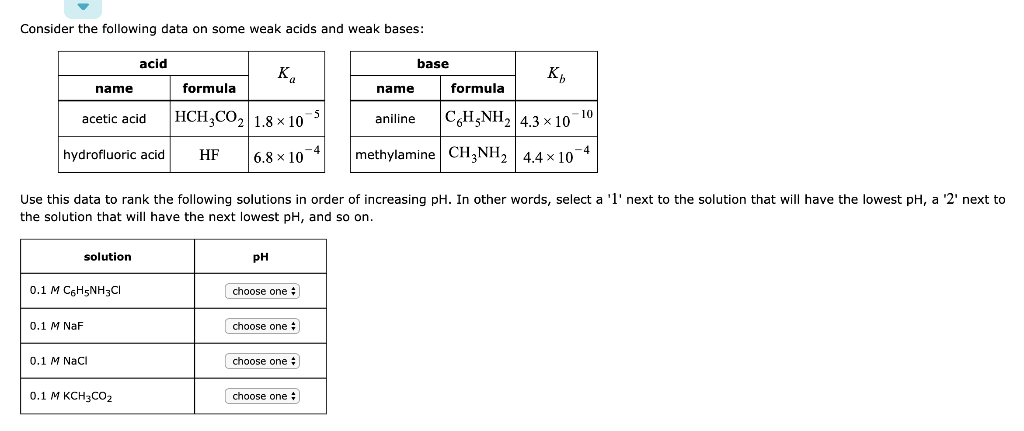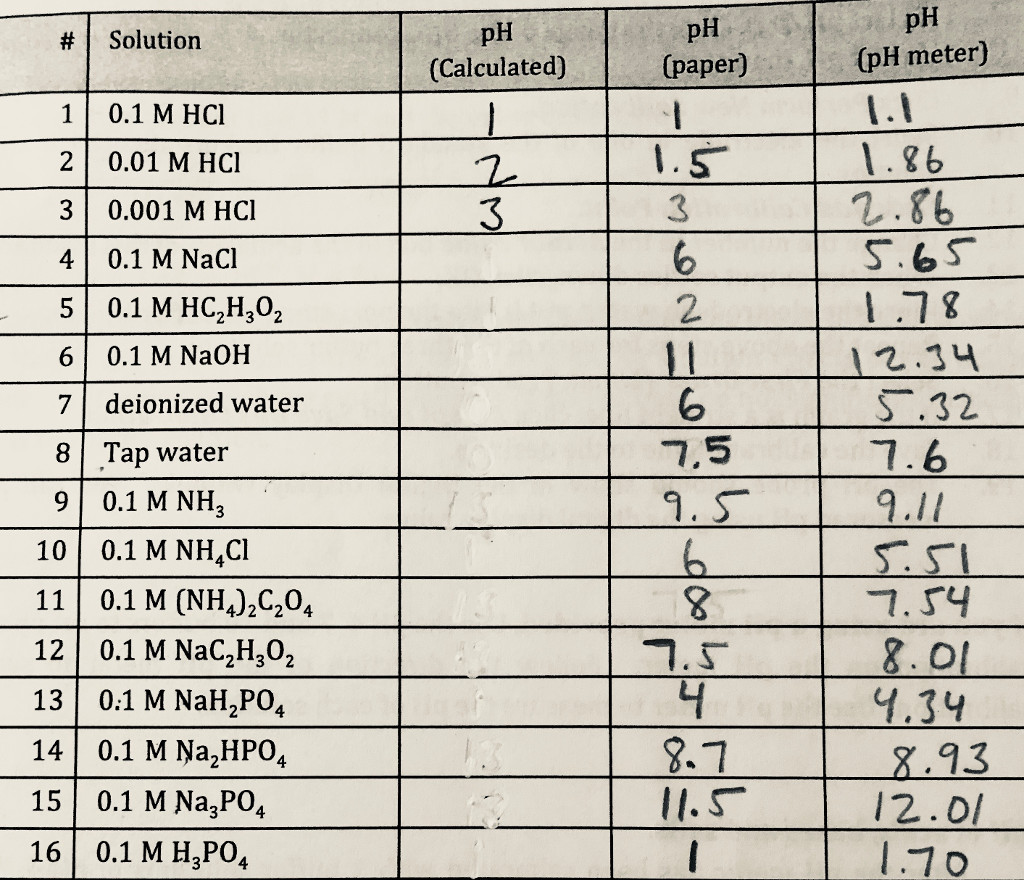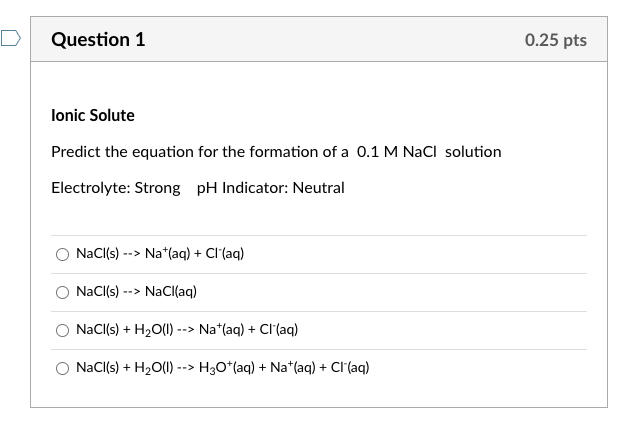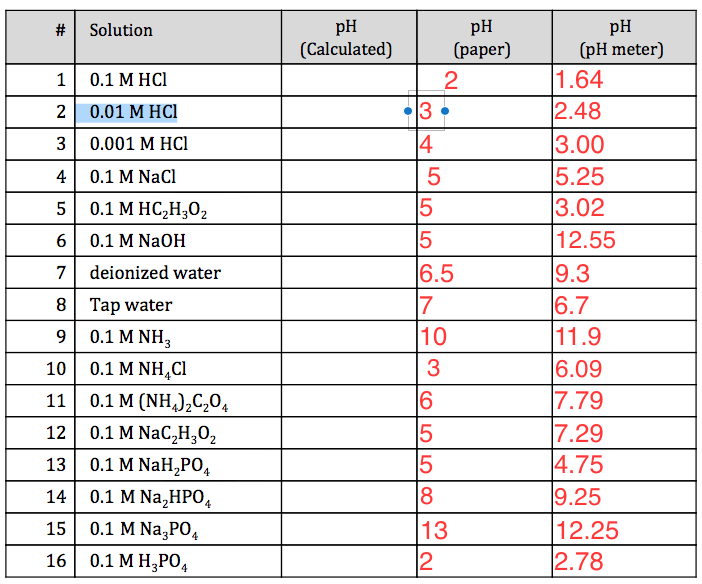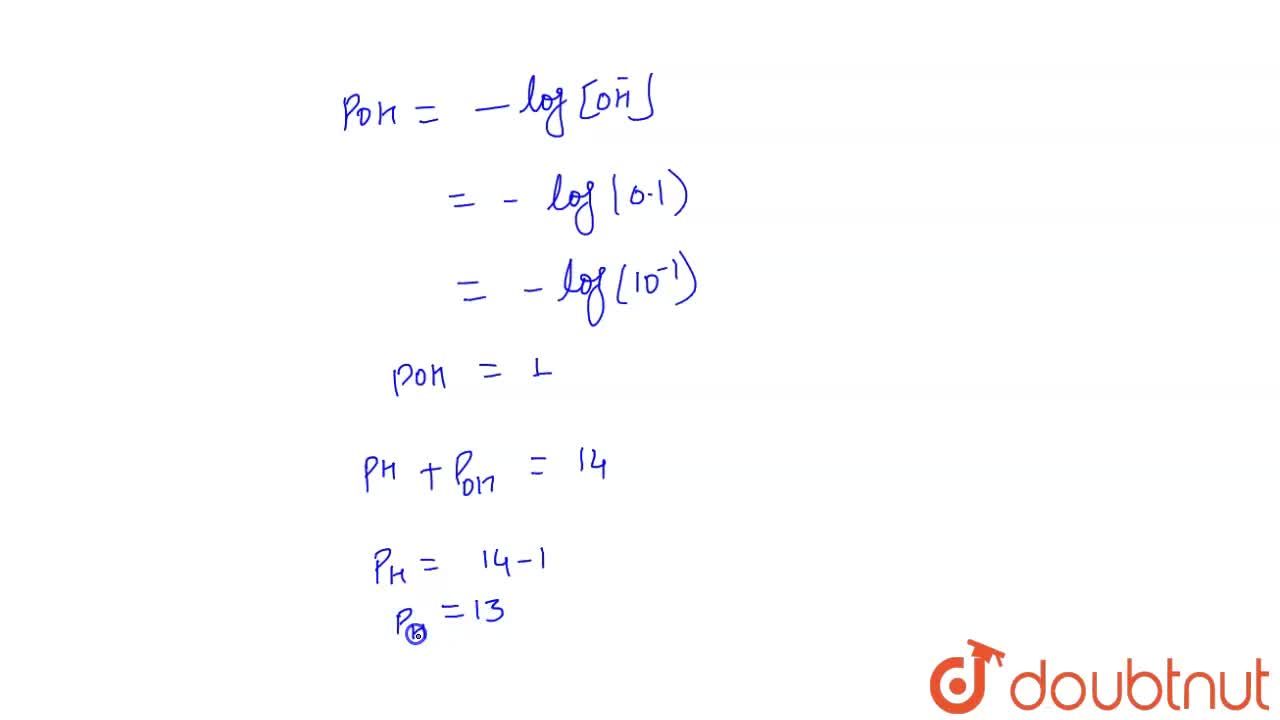
Calculate the pH of 0.5 of 1.0 M NaCl solution after electrolysis when a current of 5.0 ampere is passed for 965 seconds.
Q)In which of the following cases ph is greater than 7? a)5oml of 0.1 M HCl + 50ml of 0.1 M NaCl b)50ml of 0.1M H2SO4 + 50ml of 0.2M KOH c)50ml

Q 30 the highest pH value is of:- (1) 0 1 M NaCl - Chemistry - Chemical Kinetics - 12574793 | Meritnation.com

SOLVED: and, if necessary, adjust the pH to 7.4 (using either the 0.1 M HCL or 1.0 M NaOH solutions): Adjust the final volume to 100 mL with DDW. Save this solution
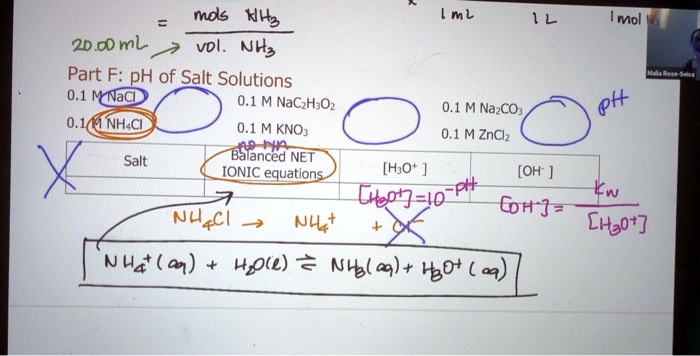
SOLVED: mds Nh? ml Mol 2D.@ml Vdl Nhs Part F: pH of Salt Solutions Nacl 0.1 M NaCzH;Oz 0.1 M NazCOz Ph NHACI 0.1 M KNO 0.1 M ZnClz Salt Balanced NET