Effect of extracellular NH3/NH4+ on intracellular pH (pHi) of a squid... | Download Scientific Diagram
Mass spectra of self-association of S1 in pH 4.5 NH4⁺ (a) and in pH 9.0... | Download Scientific Diagram
47. What is the mol of (NH4)2SO4 made for buffer solution of PH=8.26 in 0.01M NH4OH solution Playback(NH4+)=9.26 1)0.05mol 2)0.025mol 3)0.10mol 4)0.005mol

If pH of 0.1M solution of (NH4)2CO3 (aq) is x than pH of 0.01M solution of ( NH4)2CO3 (aq) would be?

Calculate the weight of (NH4)2SO4 which must be added to 500 mL of 0.2 M NH3 to yield a solution of pH = 9.35, Kb for NH3 = 1.78 × 10^-5 (in gm)(Write answer nearest integer)

Effect of pH on the removal of NH4⁺ ion by MNC (left) and vermiculite... | Download Scientific Diagram

SOLVED: Calculate the ratio [NH; [NH4 in ammonialammonium chloride buffered solutions with the following pH values: (This problem requires values in your textbook's specific appendices, which you can access through the OWLv2

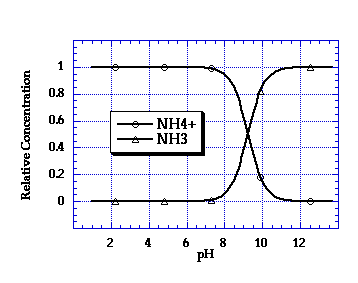
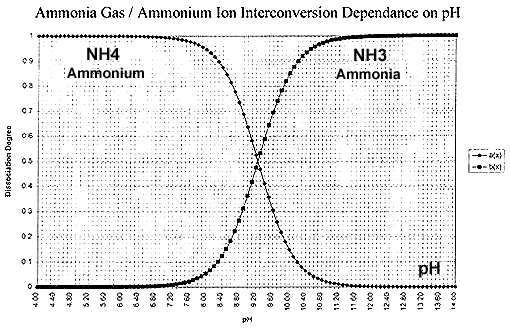
5 Relationship between the ammonia/ammonium (NH 3 /NH 4 + ) ratio and pH | Download Scientific Diagram
![The percentage of [NH 3 ] and [NH 4 + ] in total [NH X ] vs. pH. This... | Download Scientific Diagram The percentage of [NH 3 ] and [NH 4 + ] in total [NH X ] vs. pH. This... | Download Scientific Diagram](https://www.researchgate.net/publication/304071121/figure/fig2/AS:711929886687233@1546748720897/The-percentage-of-NH-3-and-NH-4-in-total-NH-X-vs-pH-This-figure-was-modified.png)